Abstract
Background
Apples are the most widely consumed fruits in Germany and various other countries. Positive health effects of apple-derived polyphenols in vivo depend on their absorption, metabolism, distribution, and elimination from the body after consumption. Data on the metabolism of these polyphenols in humans are scarce. In order to study the intestinal transit and metabolism of apple polyphenols in humans, a variety of experiments were carried out.
Methods
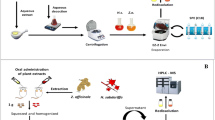
Polyphenols were incubated with saliva (for 5 min), simulated gastric or duodenal juice (4 or 10 h, respectively), or rat hepatocytes (4 h) under aerobic conditions, and with ileostomy fluid under aerobic conditions for 10 h. The polyphenol profile in human serum (8 h later) and renal elimination in urine (24 h later) were also investigated after consumption of 1 L apple juice. Polyphenols and their metabolites were identified and quantified by high-performance liquid chromatography with diode array detection (HPLC–DAD), HPLC–electrospray ionization–tandem mass spectrometry (ESI-MS/MS), and gas chromatography (GC)-MS.
Results
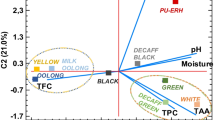
In the presence of native saliva or ileostomy fluid, β-glycosides of phloretin and quercetin were hydrolyzed, to varying degrees depending on the sugar moiety, and to much lesser degrees in the presence of antibiotics. In the gastric milieu, almost complete degradation of procyanidin B2 to (−)-epicatechin was observed. In the presence of artificial duodenal juice flavan-3-ol epimerization occurred. Quercetin was completely converted to phloroglucinol, 3,4-dihydroxybenzoic acid, and 2,4,6-trihydroxybenzoic acid. Formation of isomeric products of hydroxycinnamic acid esters and their corresponding methyl esters was also observed, and similar results were obtained after incubation with rat hepatocytes. Products of phase II metabolism, two phloretin O-glucuronides and eight (methyl) quercetin O-glucuronides, were identified in the hepatocyte samples. Following enzymatic hydrolysis, 5-caffeoylquinic acid, 4-p-coumaroylquinic acid, caffeic acid, (−)-epicatechin, phloretin, and quercetin were recovered in both serum and urine (5.3% and 3.5% of the amounts consumed, respectively). In addition, 19.5% of the polyphenols consumed were identified in the urine in the form of hydroxylated phenolic and hippuric acids.
Conclusion
The findings relating to the absorption, metabolism, and systemic availability of polyphenols in vivo should contribute to our understanding of their biological effects, and the characterization of newly formed metabolites should facilitate further studies.






Similar content being viewed by others
References
Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L (2005) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45:287–306
Verband der deutschen Fruchtsaftindustrie e.V (VdF) (2008) Daten und Fakten zur deutschen Fruchtsaft-Industrie 2007. Bonn
Wolfe K, Wu X, Liu RH (2003) Antioxidant activity of apple peels. J Agric Food Chem 51:609–614
Boyer J, Liu RH (2004) Apple phytochemicals and their health benefits. Nutr J 3:5–20
Gossé F, Guyot S, Roussi S, Lobstein A, Fischer B, Seiler NRF (2005) Chemo-preventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis 26:1291–1295
Schaefer S, Baum M, Eisenbrand G, Dietrich H, Will F, Janzowski C (2006) Polyphenolic apple juice extracts and their major constituents reduce oxidative damage in human colon cell lines. Mol Nutr Food Res 50:24–33
Veeriah S, Kautenburger T, Habermann N, Sauer J, Dietrich H, Will F, Pool-Zobel BL (2006) Apple flavonoids inhibit growth of HT29 human colon cancer cells and modulate expression of genes involved in the biotransformation of xenobiotics. Mol Carcinog 45:164–174
Oszmianski J, Wolniak M, Wojdylo A, Wawer I (2007) Comparative study of polyphenolic content and antiradical activity of cloudy and clear apple juices. J Sci Food Agric 87:573–579
Decorde K, Teissedre PL, Auger C, Cristol JP, Rouanet JM (2008) Phenolics from purple grape, apple, purple grape juice and apple juice prevent early atherosclerosis induced by an atherogenic diet in hamsters. Mol Nutr Food Res 52:400–407
Vrhovsek U, Rigo A, Tonon D, Mattivi F (2004) Quantitation of polyphenols in different apple varieties. J Agric Food Chem 52:6532–6538
Kahle K, Kraus M, Richling E (2005) Polyphenol profiles of apple juices. Mol Nutr Food Res 49:797–806
Marks SC, Mullen W, Crozier A (2007) Flavonoid and hydroxycinnamate profile of English apple ciders. J Agric Food Chem 55:8723–8730
Bergmann H, Kahle K, Triebel S, Richling E (2010) The metabolic fate of apple polyphenols in humans. Curr Nutr Food Sci 6:19–35
Kahle K, Huemmer W, Kempf M, Scheppach W, Erk T, Richling E (2007) Polyphenols are intensively metabolized in the human gastrointestinal tract after apple juice consumption. J Agric Food Chem 55:10605–10614
Gillatt PN, Palmer RC, Smith PLR, Walters CL, Reed PI (1985) Susceptibilities of drugs to nitrosation under simulated gastric conditions. Food Chem Toxic 23:849–855
Glahn RP, Lee OA, Yeung A, Goldman MI, Miller DD (1998) Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. J Nutr 128:1555–1561
Lebet V, Arrigoni E, Amadò R (1998) Measurement of fermentation products and substrate disappearance during incubation of dietary fibre sources with human faecal flora. Lebensm Wiss Technol 31:473–479
Day AJ, Bao YP, Morgan MRA, Williamson G (2000) Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic Biol Med 29:1234–1243
Kern SM, Bennett RN, Needs PW, Mellon FA, Kroon PA, Garcia-Conesa MT (2003) Characterization of metabolites of hydroxycinnamates in the in vitro model of human small intestinal epithelium Caco-2 cells. J Agric Food Chem 51:7884–7891
Kahle K, Kraus M, Scheppach W, Richling E (2005) Colonic availability of apple polyphenols–a study in ileostomy subjects. Mol Nutr Food Res 49:1143–1150
van der Woude H, Boersma MG, Vervoort J, Rietjens IM (2004) Identification of 14 quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem Res Toxicol 17:1520–1530
Macdonald IA, Mader JA, Bussard RG (1983) The role of rutin and quercitrin in stimulating flavonol glycosidase activity by cultured cell-free microbial preparations of human feces and saliva. Mutat Res 122:95–102
Hirota S, Nishioka T, Shimoda T, Miura K, Ansai T, Takahama U (2001) Quercetin glucosides are hydrolyzed to quercetin in human oral cavity to participate in peroxidase-dependent scavenging of hydrogen peroxide. Food Sci Technol Res 7:239–245
Walle T, Brownig AM, Steed LL, Reed SG, Walle UK (2005) Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J Nutr 135:48–52
Tsuchiya H, Sato M, Kato H, Okubo T, Juneja LR, Kim M (1997) Simultaneous determination of catechins in human saliva by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 703:253–258
Zhu QY, Holt RR, Lazarus SA, Ensunsa JL, Hammerstone JF, Schmitz HH, Keen CL (2002) Stability of the flavan-3-ols epicatechin and catechin and related dimeric procyanidins derived from cocoa. J Agric Food Chem 50:1700–1705
Neilson AP, Hopf AS, Cooper BR, Pereira MA, Bomser JA, Ferruzzi MG (2007) Catechin degradation with concurrent formation of homo- and heterocatechin dimers during in vitro digestion. J Agric Food Chem 55:8941–8949
Bermudéz-Soto MJ, Tomás-Barberán FA, García-Conesa MT (2007) Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem 102:865–874
Olthof MR, Hollman PC, Katan MB (2001) Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr 131:66–71
Lafay S, Gil-Izquierdo A, Manach C, Morand C, Besson C, Scalbert A (2006) Chlorogenic acid is absorbed in its intact form in the stomach of rats. J Nutr 136:1192–1197
Porter LJ (2002) Tannins. In: Harborne JB (ed) Plant biochemistry. Vol I plant phenolics. Academic Press, London, pp 389–418
Rios LY, Bennett RN, Lazarus SA, Rémésy C, Scalbert A, Williamson G (2002) Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr 76:1106–1110
Farah A, Guigon F, Trugo LC (2006) 5-Caffeoylquinic acid digestibility in human digestive fluids. Colloque Scientifique International sur le Café, 21st, Montpellier, Frankreich, 11–15, September 2006, pp 97–100
Kiatgrajai P, Wellons JD, Gollob L, White JD (1982) Kinetics of epimerization of (+)-catechin and its rearrangement to catechinic acid. J Org Chem 47:2910–2912
Hashida K, Ohara S, Makino R (2003) Base-catalyzed reactions of (−)-epicatechin: formation of enantiomers of base-catalyzed reaction products from (+)-catechin. J Food Chem Technol 23:227–232
Bergmann H, Rogoll D, Scheppach W, Melcher R, Richling E (2009) The USSING type chamber model to study the intestinal transport and modulation of specific tight-junction genes using a colonic cell line. Mol Nutr Food Res 53:1211–1225
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073S–2085S
Day AJ, Canada FJ, Diaz JC, Kroon PA, Mclauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G (2000) Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett 468:166–170
Németh K, Plumb GW, Berrin JG, Juge N, Naim HY, Williamson G, Swallow DM, Kroon PA (2003) Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr 42:29–42
Knaup B, Kahle K, Erk T, Schreier P, Richling E (2007) Human intestinal hydrolysis of phenol glycosides–a study with quercetin and p-nitrophenol glycosides using ileostomy fluid. Mol Nutr Food Res 51:1423–1429
Rasmussen SE, Frederiksen H, Krogholm KS, Poulsen L (2005) Dietary proanthocyanidins: occurence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol Nutr Food Res 49:159–174
Vaidyanathan JB, Walle T (2002) Glucuronidation and sulfation of the tea flavonoid (−)-epicatechin by the human and rat enzymes. Drug Metab Dispos 30:897–903
Moridani MY, Scobie H, O’Brien PJ (2002) Metabolism of caffeic acid by isolated rat hepatocytes and subcellular fractions. Toxicol Lett 133:141–151
Morand C, Crespy V, Manach C, Besson C, Démigné C, Rémésy C (1998) Plasma metabolites of quercetin and their antioxidant properties. Am J Physiol 275:R212–R219
Marks SC, Mullen W, Borges G, Crozier A (2009) Absorption, metabolism, and excretion of cider dihydrochalcones in healthy humans and subjects with an ileostomy. J Agric Food Chem 57:2009–2015
DuPont MS, Bennett RN, Mellon FA, Williamso G (2002) Polyphenols from alcoholic apple cider are absorbed, metabolized and excreted by humans. J Nutr 132:172–175
Cremin P, Kasim-Karakas S, Waterhouse AL (2001) LC/ES-MS detection of hydroxyl-cinnamates in human plasma and urine. J Agric Food Chem 49:1747–1750
Farah A, Monteiro M, Donangelo CM, Lafay S (2008) Chlorogenic acids from green coffee extract are highly bioavailable in humans. J Nutr 138:2309–2315
Stalmach A, Mullen W, Barron D, Uchida K, Yokota T, Cavin C, Steiling H, Williamson G, Crozier A (2009) Metabolite profiling of hydroxycinnamate derivates in plasma and urine following the ingestion of coffee by humans: identification of biomarkers of coffee consumption. Drug Metab Dispos 37:1749–1758
Renouf M, Marmet C, Guy P, Fraering AL, Longet K, Moulin J, Enslen M, Barron D, Cavin C, Dionisi F, Rezzi S, Kochhar S, Steiling H, Williamson G (2010) Nondairy creamer, but not milk, delays the appearance of coffee phenolic acid equivalents in human plasma. J Nutr 140:259–263
Adzet T, Camarasa J, Escubedu E, Merlos M (1988) In vitro study of caffeic acid-bovine serum albumin interaction. Eur J Drug Metab Pharmacokinet 13:11–14
Wittemer SM, Ploch M, Windeck T, Müller SC, Drewelow B, Derendorf H, Veit M (2005) Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of artichoke leaf extracts in humans. Phytomedicine 12:28–38
Nardini M, Cirillo E, Natella F, Scaccini C (2002) Absorption of phenolic acids in humans after coffee consumption. J Agric Food Chem 50:5735–5741
Azuma K, Ippoushi K, Nakayama M, Ito H, Higashio H, Terao J (2000) Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J Agric Food Chem 48:5496–5500
Tsang C, Auger C, Mullen W, Bornet A, Rouanet JM, Crozier A, Teissedre PL (2005) The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of grape seed extract by rats. Brit J Nutr 94:170–181
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I review of 97 bioavailability studies. Am J Clin Nutr 81:230S–242S
Mullen W, Edwards CA, Crozier A (2006) Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Brit J Nutr 96:107–116
Ito H, Gonthier MP, Manach C, Morand C, Mennen L, Rémésy C, Scalbert A (2005) Polyphenol levels in human urine after intake of six different polyphenol-rich beverages. Brit J Nutr 94:500–509
Monteiro M, Farah A, Perrone D (2007) Chlorogenic acid compounds from coffee are differentially absorbed and metabolized in humans. J Nutr 137:2196–2201
Olthof MR, Hollman PCH, Buijsman MNCP, van Amelsvoort JMM, Katan MB (2003) Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J Nutr 133:1806–1814
Clifford MN, Copeland EL, Bloxsidge JP, Mitchell LA (2000) Hippuric acid as a major excretion product associated with black tea consumption. Xenobiotica 30:317–326
Monge P, Solheim E, Scheline RR (1984) Dihydrochalcone metabolism in the rat: phloretin. Xenobiotica 14:917–924
Rios LY, Gonthier MP, Rémésy C, Mila I, Lapierre C, Lazarus SA, Williamson G, Scalbert A (2003) Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am J Clin Nutr 77:912–918
Dépréz S, Brézillon C, Rabot S, Philippe C, Mila I, Lapierre C, Scalbert A (2000) Polymeric proanthocyanidins are catabolized by human colonic microflora into low molecular weight phenolic acids. J Nutr 130:2733–2738
Ward NC, Croft KD, Puddey IB, Hodgson JM (2004) Supplementation with grape seed polyphenols results in urinary excretion of 3-hydroxyphenylpropionic acid, an important metabolite of proanthocyanidins in humans. J Agric Food Chem 52:5545–5549
Acknowledgments
We thank all the volunteers for their participation, Antje Volk and Gerda Dusel (Division of Gastroenterology, University of Wuerzburg) for taking care of the participants and their support during the study, Dr. M. Sefkow, Dr. H. Zeßner, Dr. H. Becker, and Prof. Dr. P. Winterhalter for providing polyphenol reference compounds, and S. Locher for stereoselective analysis of flavan-3-ols.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kahle, K., Kempf, M., Schreier, P. et al. Intestinal transit and systemic metabolism of apple polyphenols. Eur J Nutr 50, 507–522 (2011). https://doi.org/10.1007/s00394-010-0157-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-010-0157-0