Abstract
Aim
To investigate the effects of a neonatal low-protein diet on the morphology of myotubes in culture and the expression of key proteins that regulate myogenesis in young and adult rats.
Methods
Male Wistar rats (n = 18) were suckled by mothers fed diets containing 17% protein (controls, C) or 8% protein (undernourished, UN). All rats were fed a normal protein diet after weaning. Muscles were removed from the legs of 42-, 60- and 90-day-old rats. Muscle cells were cultured to assess cell number, morphology and the expression of major proteins involved in myogenesis (Pax7, cadherins, β1 integrin, IL-4Rα and myogenin) by western blotting. IL-4 levels in culture supernatants were measured by ELISA.
Results
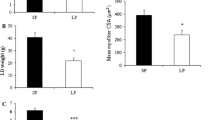
Offspring from mothers fed a low-protein diet showed a lower body weight gain. Cell number and myotube expansion were reduced in cultured muscle cells from UN, but the expression of myogenic marker proteins was unaltered.
Conclusions
Dietary restriction during lactation had no impact on the synthesis of myogenic marker proteins, and myocyte differentiation occurred normally in the muscles of offspring aged 42, 60 or 90 days. Nevertheless, the number and morphology of the myotubes are altered.




Similar content being viewed by others
References
Waterland RA, Jirtle RL (2004) Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 20:63–68
Lucas A (2005) Long-term programming effects of early nutrition—implications for the preterm infant. J Perinatol 25(Suppl 2):S2–S6
Kind KL, Simonetta G, Clifton PM, Robinson JS, Owens JA (2002) Effect of maternal feed restriction on blood pressure in the adult guinea pig. Exp Physiol 87:469–477
Ozanne SE, Hales CN (2002) Early programming of glucose-insulin metabolism. Trends Endocrinol Metab 13:368–373
Hales CN, Barker DJ (1992) Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35:595–601
Ravelli GP, Stein ZA, Susser MW (1976) Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 295:349–353
Sawaya AL, Martins PA, Grillo LP, Florencio TT (2004) Long-term effects of early malnutrition on body weight regulation. Nutr Rev 62:S127–S133
Brameld JM (2004) The influence of undernutrition on skeletal muscle development. Br J Nutr 91:327–328
Mallinson JE, Sculley DV, Craigon J, Plant R, Langley-Evans SC, Brameld JM (2007) Fetal exposure to a maternal low-protein diet during mid-gestation results in muscle-specific effects on fibre type composition in young rats. Br J Nutr 98:292–299
Wilson SJ, Ross JJ, Harris AJ (1988) A critical period for formation of secondary myotubes defined by prenatal undernourishment in rats. Development 102:815–821
Bayol S, Jones D, Goldspink G, Stickland NC (2004) The influence of undernutrition during gestation on skeletal muscle cellularity and on the expression of genes that control muscle growth. Br J Nutr 91:331–339
Perdiguero E, Sousa-Victor P, Ballestar E, Munoz-Canoves P (2009) Epigenetic regulation of myogenesis. Epigenetics 4:541–550
Wrobel E, Brzoska E, Moraczewski J (2007) M-cadherin and beta-catenin participate in differentiation of rat satellite cells. Eur J Cell Biol 86:99–109
Horsley V, Jansen KM, Mills ST, Pavlath GK (2003) IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113:483–494
Hirabara SM, Silveira LR, Alberici LC, Leandro CV, Lambertucci RH, Polimeno GC, Cury Boaventura MF, Procopio J, Vercesi AE, Curi R (2006) Acute effect of fatty acids on metabolism and mitochondrial coupling in skeletal muscle. Biochim Biophys Acta 1757:57–66
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Barker DJ (2007) The origins of the developmental origins theory. J Intern Med 261:412–417
Sayer AA, Cooper C (2005) Fetal programming of body composition and musculoskeletal development. Early Hum Dev 81:735–744
Galibois I, Parent G, Savoie L (1987) Effect of dietary proteins on time-dependent changes in plasma amino acid levels and on liver protein synthesis in rats. J Nutr 117:2027–2035
Pires-de-Melo IH, Wanderley Dos Reis F, Luz LS, Paz ST, Silva HJ, Souza SL, Leandro CG (2009) Short- and long-term effects of a neonatal low-protein diet in rats on the morphology of the larynx. Nutrition 25:855–860
Wilson MR, Hughes SJ (1997) The effect of maternal protein deficiency during pregnancy and lactation on glucose tolerance and pancreatic islet function in adult rat offspring. J Endocrinol 154:177–185
Passos MC, da Fonte Ramos C, Dutra SC, Mouco T, de Moura EG (2002) Long-term effects of malnutrition during lactation on the thyroid function of offspring. Horm Metab Res 34:40–43
Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA (1993) IGF-I is required for normal embryonic growth in mice. Genes Dev 7:2609–2617
Dwyer CM, Stickland NC, Fletcher JM (1994) The influence of maternal nutrition on muscle fiber number development in the porcine fetus and on subsequent postnatal growth. J Anim Sci 72:911–917
Barton-Davis ER, Shoturma DI, Sweeney HL (1999) Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand 167:301–305
Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, Beauchamp JR (2006) Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci 119:1824–1832
Hollnagel A, Grund C, Franke WW, Arnold HH (2002) The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Mol Cell Biol 22:4760–4770
Bozyczko D, Decker C, Muschler J, Horwitz AF (1989) Integrin on developing and adult skeletal muscle. Exp Cell Res 183:72–91
Crawley S, Farrell EM, Wang W, Gu M, Huang HY, Huynh V, Hodges BL, Cooper DN, Kaufman SJ (1997) The alpha7beta1 integrin mediates adhesion and migration of skeletal myoblasts on laminin. Exp Cell Res 235:274–286
Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U (2003) Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell 4:673–685
Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA (2009) Focal adhesion kinase signaling regulates the expression of caveolin 3 and beta1 integrin, genes essential for normal myoblast fusion. Mol Biol Cell 20:3422–3435
Acknowledgments
The authors wish to thank CAPES-COFECUB (grant 584/07) and the National Council for Science and Technology (CNPq), Brazil, for their financial support.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Melo, J.F., Aloulou, N., Duval, JL. et al. Effect of a neonatal low-protein diet on the morphology of myotubes in culture and the expression of key proteins that regulate myogenesis in young and adult rats. Eur J Nutr 50, 243–250 (2011). https://doi.org/10.1007/s00394-010-0132-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-010-0132-9