Abstract
Background
A low folate status and mitochondrial DNA (mtDNA) mutations are risk factors for various cancers and degenerative diseases. It is not known if lymphocytic mtDNA deletions can be used as genetic “markers” to reflect global mtDNA damage during folate deficiency.
Aim of the study
The aim of this study was to characterize folate-related mtDNA deletions in lymphocytes and their associations with mt genotoxicity in peripheral tissues.
Methods
Weaning Wistar rats were fed folate-deficient and folate-replete (control) diets for 2 and 4 weeks. Folate levels of blood lymphocytes and various tissues were assayed by the Lactobacillus casei method. mtDNA deletions were measured by a real-time polymerase chain reaction analysis of whole DNA extracts.
Results
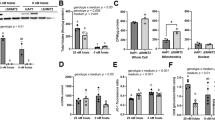
Compared to the control counterparts, mtDNA deletions of lymphocytes increased by 3.5-fold (P < 0.05) after 4 weeks of folate deficiency. Lymphocytic mtDNA deletions were inversely associated with plasma (r = −0.619, P = 0.018), red blood cell (r = −0.668, P = 0.009), and lymphocytic folate levels (r = −0.536, P = 0.048). Frequencies of lymphatic mtDNA deletions were positively correlated with mtDNA deletions in tissues including the lungs (r = 0.803, P = 0.0005), muscles (r = 0.755, P = 0.001), heart (r = 0.633, P = 0.015), liver (r = 0.722, P = 0.003), kidneys (r = 0.737, P = 0.006), pancreas (r = 0.666, P = 0.009), and brain (r = 0.917, P < 0.0001). Conclusions: Our data demonstrate that accumulated mtDNA deletions of lymphocytes depended upon dietary folate deprivation. The accumulated mt deletions in lymphocytes closely reflected the mt genotoxicity in the peripheral tissues during folate deficiency.


Similar content being viewed by others
Abbreviations
- FD:
-
Folate-deficient
- mt:
-
Mitochondrial
- mtDNA4834 deletion:
-
4,834-bp large deletion in mtDNA
- ROS:
-
Reactive oxygen species
- RE:
-
Relative expression
References
Biagini G, Pallotti F, Carraro S, Sgarbi G, Pich MM, Lenaz G, Anzivino F, Gualandi G, Xin D (1998) Mitochondrial DNA in platelets from aged subjects. Mech Ageing Dev 101:254–269
Branda RF, Brooks EM, Chen Z, Naud SJ, Nicklas JA (2002) Dietary modulation of mitochondrial DNA deletions and copy number after chemotherapy in rats. Mutat Res 501:29–36
Chang CM, Yu CC, Lu HT, Chou YF, Huang RFS (2007) Folate deprivation promotes mitochondrial oxidative decay: DNA large deletions, cytochrome c oxidase dysfunction, membrane depolarization. Br J Nutr 97:855–863
Choi SW, Mason JB (2000) Folate and carcinogenesis: an integrated scheme. J Nutr 130:129–135
Chou YF, Yu CC, Huang RFS (2007) Changes in mitochondrial (mt) DNA deletion, content and biogenesis in folate-deficient tissues of young rats depend on mt folate and oxidative DNA injuries. J Nutr 100:596–602
Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC (1992) Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet 2:324–398
Cortopassi GA, Shibata DD, Soong NW, Arnheim N (1992) A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc Natl Acad Sci USA 89:7370–7374
Crott JW, Choi SW, Branda RF, Mason JB (2002) Accumulation of mitochondrial DNA deletions is age, tissue and folate-dependent in rats. Mutat Res 570:63–70
Doria G, Frasca D (2001) Age-related changes of DNA damage and repair capacity in cells of the immune system. Mech Ageing Dev 122:985–998
Duthie SJ, Grant G, Narayanan S (2001) Increased uracil misincorporation in lymphocytes from folate-deficient rats. Br J Cancer 83:1532–1537
Fenech M (2001) The role of folic acid and vitamin B12 in genomic stability of human cells. Mutat Res 475:51–67
Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D (2000) Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science 287:2017–2019
Holt IJ, Harding AE, Morgan-Hughes JA (1998) Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 331:717–719
Horne DW, Patterson D (1988) Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem 34:2357–2359
Huang RFS, Hsu YC, Lin HL, Yang FL (2001) Folate depletion and elevated plasma homocysteine promote oxidative stress in rat livers. J Nutr 131:33–38
Hultenby K, Rustin P, Gustafsson CM, Larsson NG (2004) Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet 13:935–944
Jeronimo C, Nomoto S, Caballero OL, Usadel H, Henrique R, Varzim G, Oliveira J, Lopes C, Fliss M, Sidransky D (2001) Mitochondrial mutations in early stage prostate cancer and bodily fluids. Oncogene 20:5195–5198
Kim UK, Kim HS, Oh BH, Lee MM, Kim SH, Chae JJ, Choi HS, Choe SC, Lee CC, Park YB (2000) Analysis of mitochondrial DNA deletions in four chambers of failing human heart: hemodynamic stress, age, and disease are important factors. Basic Res Cardiol 95:163–171
Kopxidas G, Kovalenko SA, Heffernan DR, Yarovaya N, Kramarova L, Stojanoveki D, Borg J, Islam MM, Caragounis A, Linnane AW (2000) Tissue mitochondrial DNA changes. Ann NY Acad Sci 908:226–243
Kuo CS, Lin CY, Wu MY, Lu CL, Huang RF (2008) Relationship between folate status and tumour progression in patients with hepatocellular carcinoma. Br J Nutr 100:596–602
Lee HC, Pang CY, Hsu HS, Wei YH (1994) Differential accumulations of 4977 bp deletion in mitochondrial DNA of various tissues in human ageing. Biochim Biophys Acta 1226:37–43
Lestienne P, Ponsot G (1988) Kearns–Sayre syndrome with muscle mitochondrial deletion. Lancet 1:885
Loscalzo J (1996) The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest 98:5–7
Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R (2007) Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Arch Neurol 64:86–92
Mandel HR, Szargel V, Labay O, Elpereg A, Saada A, Shalata Y, Anbinder D, Berkowich C, Barak HM (2001) The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocellular mitochondrial DNA. Nat Genet 29:337
Meissner C, Mohamed SA, Klueter H, Hamann K, von Wurmb N, Oehmichen M (2000) Quantification of mitochondrial DNA in human blood cells using an automated detection system. Forensic Sci Int 113:109–112
Melberg A, Nennesmo I, Moslemi AR, Kollberg G, Luoma P, Suomalainen A, Holme E, Oldfors A (2005) Alzheimer pathology associated with POLG1 mutation, multiple mtDNA deletions, and APOE4/4: premature ageing or just coincidence? Acta Neuropathol 110:315–316
Mohamed SA, Wesch D, Blumenthal A, Bruse P, Windler K, Ernst M, Kabelitz D, Oehmichen M, Meissner C (2004) Detection of the 4977 bp deletion of mitochondrial DNA in different human blood cells. Exp Gerontol 39:181–188
Moraes CT, DiMauro S, Zeviani M, Lombes A, Shanske S, Miranda AF, Nakase H, Bonilla E, Werneck LC (1989) Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns–Sayre syndrome. N Engl J Med 320:1293–1299
Raha S, Robinson BH (2000) Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci 25:502–583
Rampersaud GC, Kauwell GP, Hutson AD, Cerda JJ, Bailey LB (2000) Genomic DNA methylation decreases in response to moderate folate depletion in elderly women. Am J Clin Nutr 72:998–1003
Ravagnan L, Roumier T, Kroemer G (2002) Mitochondria, the killer organelles and their weapons. J Cell Physiol 192:131–137
Ross OA, Hyland P, Curran MD, McIlhatton BP, Wikby A, Johansson B, Tompa A, Rawelec G, Barnett CR, Middleton D, Barnett YA (2002) Mitochondrial DNA damage in lymphocytes: a role in immunosenescence? Exp Gerontol 37:329–340
Varela-Moreiras G, Selhub J (1992) Long-term folate deficiency alters folate content and distribution differentially in rat tissues. J Nutr 122:986–991
Virbasius JV, Scarpulla RC (1994) Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci USA 91:1309–1313
Von Wurmb N, Oehmichen M, Meissner C (1998) Demonstration of the 4977 bp deletion in human mitochondrial DNA from intravital and postmortem blood. Mutat Res 422:247–254
Walzem RL, Clifford AJ (1988) Folate deficiency in rats fed diets containing free amino acid or intact proteins. J Nutr 118:1089–1096
Wang Y, Michikawa Y, Mallidis C, Bai Y, Woodhouse L, Yarasheski KE, Miller CA, Askanas V, Engel WK, Bhasin S, Attardi G (2007) Muscle-specific mutations accumulate with aging in critical human mtDNA control sites for replication. Proc Natl Acad Sci USA 98:4022–4027
Wei YH (1992) Mitochondrial DNA alterations as ageing-associated molecular events. Mutat Res 275:145–155
Wei YH, Lee HC (2002) Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 227:671–682
Wei YH (1998) Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med 217:53–63
Wei YH, Pang CY, Lee HC, Lu CY (1998) Roles of mitochondrial DNA mutation and oxidative damage in human aging. Curr Sci 74:887–893
Yin PH, Lee HC, Chau GY, Wu YT, Li SH, Liu WY, Wei YH, Liu TY, Chi CW (2004) Alteration of the copy number and deletion of mitochondrial DNA in human hepatocellular carcinoma. Br J Cancer 90:2390–2392
Acknowledgments
The authors are deeply grateful to Prof. Y.-H. Wei of the Department of Biochemistry at National Yang-Ming University, Taipei, Taiwan for his kind support and invaluable advice on mtDNA mutations. This study was supported by a grant (NSC96-2320-B-002) from the National Science Council, Taiwan to R.F.S. Huang.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chou, YF., Huang, RF.S. Mitochondrial DNA deletions of blood lymphocytes as genetic markers of low folate-related mitochondrial genotoxicity in peripheral tissues. Eur J Nutr 48, 429–436 (2009). https://doi.org/10.1007/s00394-009-0031-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-009-0031-0