Abstract
Objective
To examine whether alcohol intake is causally associated with rheumatoid arthritis (RA).
Methods
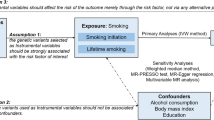
We performed a two-sample Mendelian randomization (MR) analysis using the inverse-variance weighted (IVW), weighted median, and MR-Egger regression methods. We used the publicly available summary statistics of alcohol intake frequency from the UK Biobank genome-wide association studies (GWASs; n = 336,965) as the exposure and a GWAS meta-analysis of 5539 autoantibody-positive RA patients and 20,169 controls as the outcome.
Results
We selected 24 single nucleotide polymorphisms (SNPs) associated with alcohol intake frequency at genome-wide significance as instrumental variables (IVs) to improve inference, 16 of which were inversely associated with RA. The IVW method showed no evidence of a causal association between alcohol intake and RA (beta = 0.218, SE = 0.213, p = 0.306). The MR-Egger regression revealed that directional pleiotropy was unlikely to bias the result (intercept = 0.027, p = 0.292). The MR-Egger analysis and the weighted median approach showed no causal association between alcohol intake and RA (beta = −0.778, SE = 0.947, p = 0.420 and beta = −0.286, SE = 0.302, p = 0.344, respectively). Cochran’s Q test did not indicate heterogeneity between IV estimates based on the individual variants, and results from a “leave-one-out” analysis demonstrated that no single SNP was driving the IVW point estimate.
Conclusion
The MR analysis does not support a causal inverse association between alcohol intake and RA occurrence.
Zusammenfassung
Ziel
In der vorliegenden Studie wurde untersucht, ob Alkoholkonsum kausal mit der rheumatoiden Arthritis (RA) zusammenhängt.
Methoden
Es wurde eine Zwei-Stichproben-Mendel-Randomisierungs(MR)-Analyse mit Inverse-Varianz-Gewichtung (IVG), gewichtetem Median und MR-Egger-Regression durchgeführt. Dafür herangezogen wurden die öffentlich zugänglichen statistischen Kennzahlen zur Häufigkeit des Alkoholkonsums aus den genomweiten Assoziationsstudien (GWAS) der UK Biobank (n = 336.965) für die Exposition sowie eine GWAS-Metaanalyse von 5539 Autoantikörper-positiven Patienten mit RA und 20.169 Kontrollpersonen für das Outcome.
Ergebnisse
Insgesamt 24 Einzelnukleotidpolymorphismen (SNP), die mit genomweiter Signifikanz mit der Häufigkeit des Alkoholkonsums assoziiert waren, wurden als Instrumentvariablen (IV) ausgewählt, um bessere Schlussfolgerungen zu ermöglichen. Von diesen waren 16 invers mit RA assoziiert. Die IVG-Methode ergab keinen Hinweis auf einen kausalen Zusammenhang zwischen Alkoholkonsum und RA (beta = 0,218, SE = 0,213, p = 0,306). Die MR-Egger-Regression zeigte, dass eine Verzerrung des Ergebnisses durch eine gerichtete Pleiotropie unwahrscheinlich war (Achsenabschnitt =0,027, p = 0,292). Die MR-Egger-Analyse und der Ansatz mit gewichtetem Median ergaben keinen kausalen Zusammenhang zwischen Alkoholkonsum und RA (beta = −0,778, SE = 0,947, p = 0,420 bzw. beta = −0,286, SE = 0,302, p = 0,344). Der Cochran-Q-Test wies nicht auf eine Heterogenität zwischen IV-Schätzungen auf Grundlage der individuellen Varianten hin, und eine Leave-one-out-Analyse zeigte, dass nicht ein einzelner SNP die IVG-Punktschätzung bestimmte.
Schlussfolgerung
Die MR-Analyse stützt eine kausale inverse Assoziation zwischen Alkoholkonsum und dem Auftreten einer RA nicht.


Similar content being viewed by others
References
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44(2):512–525
Bowden J, Davey Smith G, Haycock PC et al (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40(4):304–314
Bowden J, Del Greco F, Minelli C et al (2016) Assessing the suitability of summary data for Mendelian randomization analyses using MR-Egger regression: the role of the I^ 2 statistic. Int J Epidemiol 45(6):1961–1974
Brion M-JA, Shakhbazov K, Visscher PM (2012) Calculating statistical power in Mendelian randomization studies. International journal of epidemiology 42(5):1497–1501
Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37(7):658–665
Burgess S, Daniel RM, Butterworth AS et al (2014) Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol 44(2):484–495
Burgess S, Dudbridge F, Thompson SG (2016) Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med 35(11):1880–1906
Burgess S, Thompson SG (2017) Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 32(5):377–389
Edwards C, Cooper C (2006) Early environmental factors and rheumatoid arthritis. Clinical & Experimental. Immunology 143(1):1–5
Egger M, Smith GD, Phillips AN (1997) Meta-analysis: principles and procedures. BMJ 315(7121):1533–1537
Hartwig FP, Davies NM, Hemani G et al (2016) Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol 45(6):1717–1726
Hemani G, Zheng J (2016) Wade K et al MR-Base: a platform for systematic causal inference across the phenome using billions of genetic associations. bioRxiv. Acta Neurochir (Wien) 16:78972 (Dec)
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Cochran WG, Rubin DB (1973) Controlling bias in observational studies: A review. Sankhyā: Indian J Stat Series A:417–446
Jin Z, Xiang C, Cai Q et al (2014) Alcohol consumption as a preventive factor for developing rheumatoid arthritis: a dose-response meta-analysis of prospective studies. Ann Rheum Dis 73(11):1962–1967
Jonsson M, Verdrengh M, Brisslert M et al (2007) Ethanol prevents development of destructive arthritis. Proc Natl Acad Sci 104(1):258–263
Lawlor DA (2016) Commentary: Two-sample Mendelian randomization: opportunities and challenges. Int J Epidemiol 45(3):908
Lee YH, Bae S‑C, Choi SJ et al (2012) Genome-wide pathway analysis of genome-wide association studies on systemic lupus erythematosus and rheumatoid arthritis. Mol Biol Rep 39(12):10627–10635
Lee YH, Bae SC, Song GG (2013) Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occultcarriers) undergoing antitumor necrosis factor therapy. Clin Exp Rheumatol 31(1):118–121
Mandrekar P, Jeliazkova V, Catalano D et al (2007) Acute alcohol exposure exerts anti-inflammatory effects by inhibiting IκB kinase activity and p65 phosphorylation in human monocytes. J Immunol 178(12):7686–7693
Pierce BL, Burgess S (2013) Efficient design for Mendelian randomization studies: subsample and 2‑sample instrumental variable estimators. Am J Epidemiol 178(7):1177–1184
Scott IC, Tan R, Stahl D et al (2013) The protective effect of alcohol on developing rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology 52(5):856–867
Smith GD, Ebrahim S (2003) ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease?. Int J Epidemiolog 32(1):1–22
Smith GD, Ebrahim S (2004) Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol 33(1):30–42
Stahl EA, Raychaudhuri S, Remmers EF et al (2010) Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 42(6):508
Swerdlow DI, Kuchenbaecker KB, Shah S et al (2016) Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int J Epidemiol 45(5):1600–1616
Thompson JR, Minelli C, Bowden J et al (2017) Mendelian randomization incorporating uncertainty about pleiotropy. Stat Med 36(29):4627–4645
Waldschmidt TJ, Cook RT, Kovacs EJ (2008) Alcohol and inflammation and immune responses: summary of the 2006 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol 42(2):137–142
Acknowledgements
This study was supported in part by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (HI15C2958).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.-C. Bae and Y. H. Lee declare that they have no competing interests.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Redaktion
U. Müller-Ladner, Bad Nauheim
U. Lange, Bad Nauheim
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Bae, S., Lee, Y.H. Alcohol intake and risk of rheumatoid arthritis: a Mendelian randomization study. Z Rheumatol 78, 791–796 (2019). https://doi.org/10.1007/s00393-018-0537-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-018-0537-z
Keywords
- Alcohol intake
- Rheumatoid arthritis
- Mendelian randomization
- Genetic predisposition to disease
- Genome-wide association study