Abstract
Objective
To assess the relative efficacy and safety of apremilast, secukinumab, and ustekinumab at different doses in patients with active psoriatic arthritis (PsA).
Method
A Bayesian network meta-analysis was conducted, which included randomized controlled trials (RCTs) that examined the efficacy and safety of apremilast 20 mg, apremilast 30 mg, secukinumab 75 mg, secukinumab 150 mg, secukinumab 300 mg, ustekinumab 45 mg, and ustekinumab 90 mg compared with placebo.
Results
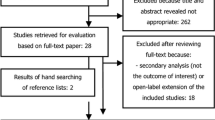
Of the RCTs 8 comprising 3289 patients met the inclusion criteria. The American College of Rheumatology (ACR) 20 response rate was significantly higher in the secukinumab 300 mg group than in the placebo group (odds ratio OR, 7.55; 95% confidence interval CI, 3.18–17.63). Secukinumab 150 mg, secukinumab 75 mg, ustekinumab 90 mg, apremilast 30 mg, apremilast 20 mg, and ustekinumab 45 mg were also more efficacious than placebo. There were no significant differences in the efficacy between the interventions. A dose-response relationship among the same drug groups was observed. The number of serious adverse events was not significantly different among the apremilast, secukinumab, ustekinumab, and placebo groups.
Conclusion
All drug treatments were more efficacious than placebo; however, there were no significant differences in the efficacy and safety between the drugs at the different doses.
Zusammenfassung
Ziel
Ziel dieser Studie war es, die relative Wirksamkeit und Sicherheit von Apremilast, Secukinumab und Ustekinumab in unterschiedlichen Dosierungen bei Patienten mit aktiver Psoriasisarthritis (PsA) zu untersuchen.
Methode
Eine Bayessche-Netzwerk-Metaanalyse wurde durchgeführt, die randomisierte kontrollierte Studien (RCT) einschloss, welche die Wirksamkeit und Sicherheit von Apremilast 20 mg, Apremilast 30 mg, Secukinumab 75 mg, Secukinumab 150 mg, Secukinumab 300 mg, Ustekinumab 45 mg und Ustekinumab 90 mg verglichen mit Placebo untersuchten.
Ergebnisse
Von den RCT erfüllten 8 Studien mit 3289 Patienten die Einschlusskriterien. Die Ansprechrate nach den Kriterien des American College of Rheumatology (ACR) 20 war signifikant höher in der Secukinumab-300-mg-Gruppe als in der Placebogruppe (Odds-Ratio: 7,55; 95% Konfidenzintervall [CI] 3,18–17,63). Secukinumab 150 mg, Secukinumab 75 mg, Ustekinumab 90 mg, Apremilast 30 mg, Apremilast 20 mg und Ustekinumab 45 mg waren ebenfalls wirksamer als Placebo. Bei den Interventionen gab es keine signifikanten Unterschiede bezüglich der Wirksamkeit. Unter den gleichen Arzneimittelgruppen wurde eine Dosis-Wirkungs-Beziehung beobachtet. Die Anzahl schwerer unerwünschter Ereignisse unterschied sich in den Gruppen mit Apremilast, Secukinumab, Ustekinumab und Placebo nicht signifikant.
Schlussfolgerung
Alle medikamentösen Behandlungen waren wirksamer als Placebo; jedoch gab es keine signifikanten Unterschiede bezüglich der Wirksamkeit und Sicherheit bei den Arzneimitteln in unterschiedlichen Dosierungen.



Similar content being viewed by others
References
Gladman D, Antoni C, Mease P, Clegg D, Nash P (2005) Psoriatic arthritis and psoriasis: classification, clinical features, pathophysiology, immunology, genetics-psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 64:II14
Ash Z, Gaujoux-Viala C, Gossec L, Hensor EM, FitzGerald O, Winthrop K, Van Der Heijde D, Emery P, Smolen JS, Marzo-Ortega H (2011) A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 71(3):319–326. doi:10.1136/ard.2011.150995
Gossec L, Smolen J, Gaujoux-Viala C, Ash Z, Marzo-Ortega H, Van Der Heijde D, FitzGerald O, Aletaha D, Balint P, Boumpas D (2012) European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis 71:4–12
Schafer P (2012) Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol 83:1583–1590
Houslay MD, Schafer P, Zhang KY (2005) Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today 10:1503–1519
Conti M, Beavo J (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B (2014) Secukinumab in plaque psoriasis – results of two phase 3 trials. New Engl J Med 371:326–338
Kirkham BW, Kavanaugh A, Reich K (2014) Interleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology 141:133–142
Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB, Investigators PS (2008) Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371:1665–1674
Ritchlin C, Haas-Smith S, Hicks D, Cappuccio J, Osterland C, Looney R (1998) Patterns of cytokine production in psoriatic synovium. J Rheumatol 25:1544–1552
Lee YH, Bae SC, Choi SJ, Ji JD (2010) Song GG Associations between vitamin D receptor polymorphisms and susceptibility to rheumatoid arthritis and systemic lupus erythematosus: a meta-analysis. Mol Biol Rep 38(6):3643–3651. doi:10.1007/s11033-010-0477-4
Lee YH, Rho YH, Choi SJ, Ji JD, Song GG (2007) PADI4 polymorphisms and rheumatoid arthritis susceptibility: a meta-analysis. Rheumatol Int 27:827–833. doi:10.1007/s00296-007-0320-y
Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B (2014) Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int 34:1489–1496
Caldwell DM, Ades A, Higgins J (2005) Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331:897
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269
Brown S, Hutton B, Clifford T, Coyle D, Grima D, Wells G, Cameron C (2014) A Microsoft-Excel-Based tool for running and critically appraising network meta-analyses – an overview and application of netMetaXL. Syst Rev 3:110
Sutton AJ, Abrams KR (2001) Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res 10:277–303
Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP (2012) Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 41:818–827
Salanti G, Ades A, Ioannidis JP (2011) Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 64:163–171
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades A (2013) Evidence synthesis for decision making 4 inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 33:641–656
Higgins J, Jackson D, Barrett J, Lu G, Ades A, White I (2012) Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 3:98–110
Valkenhoef G, Lu G, Brock B, Hillege H, Ades A, Welton NJ (2012) Automating network meta-analysis. Res Synth Methods 3:285–299
Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, Lespessailles E, Hall S, Hochfeld M, Hu C (2014) Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 73:1020–1026
Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey AR, Hu C, Stevens R, de Vlam KL (2012) Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 64:3156–3167
McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, Van der Heijde D, Landewé R, Conaghan PG, Gottlieb AB (2015) Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386:1137–1146
Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, Van Der Heijde D, Landewé R, Nash P, Pricop L, Yuan J (2015) Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. New Engl J Med 373:1329–1339
Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, Wang Y, Shen Y‑K, Doyle MK, Mendelsohn AM (2014) Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6‑month and 1‑year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 73(6):990–999. doi:10.1136/annrheumdis-2013-204655
McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM (2013) Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382:780–789
Gottlieb A, Menter A, Mendelsohn A, Shen Y‑K, Li S, Guzzo C, Fretzin S, Kunynetz R, Kavanaugh A (2009) Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 373:633–640
Edwards CJ, Blanco FJ, Crowley J, Birbara CA, Jaworski J, Aelion J, Stevens RM, Vessey A, Zhan X, Bird P (2016) Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis 75(6):1065–1073. doi:10.1136/annrheumdis-2015-207963
Bhatnagar N, Lakshmi PV, Jeyashree K (2014) Multiple treatment and indirect treatment comparisons: an overview of network meta-analysis. Perspect Clin Res 5:154–158
Mavridis D, Giannatsi M, Cipriani A, Salanti G (2015) A primer on network meta-analysis with emphasis on mental health. Evid Based Ment Health 18:40–46
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162:777–784
Ungprasert P, Thongprayoon C, Davis JM III (2016) Indirect comparisons of the efficacy of subsequent biological agents in patients with psoriatic arthritis with an inadequate response to tumor necrosis factor inhibitors: a meta-analysis. Clin Rheumatol 35:1795–1803
Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR (2005) How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Stat Med 24:2401–2428
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G.G. Song and Y.H. Lee declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Redaktion
U. Müller-Ladner, Bad Nauheim
U. Lange, Bad Nauheim
Rights and permissions
About this article
Cite this article
Song, G.G., Lee, Y.H. Relative efficacy and safety of apremilast, secukinumab, and ustekinumab for the treatment of psoriatic arthritis. Z Rheumatol 77, 613–620 (2018). https://doi.org/10.1007/s00393-017-0355-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-017-0355-8