Abstract
Background
Systemic lupus erythematosus (SLE) is a common complex disease characterized by chronic generalized inflammation which may involve several tissues and organs.
Objective
The aim of this work was to study the expression of Toll-like receptors (TLR) 3 and 9 in SLE patients, and to investigate their relationship to clinical features, disease activity, and damage.
Patients and methods
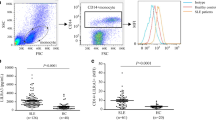
The current study included 24 Egyptian female SLE patients and 15 matched controls. Disease activity was assessed using the SLE Disease Activity Index (SLEDAI) and damage using the Systemic Lupus International Collaborating Clinics (SLICC) index. Expression of TLR3 and TLR9 in B- (CD19-positive) and T-lymphocytes (CD3-positive) was studied using flow cytometry.
Results
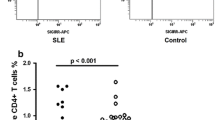
Patient age ranged between 17 and 42 years (mean 26.17 ± 5.78 years). There was a significant difference between patients and controls regarding TLR3/CD3, TLR3/CD19, TLR9/CD3, and TLR9/CD19 expression (p < 0.0001). There were significant correlations of TLR3/CD3, TLR3/CD19, and TLR9/CD19 with serum creatinine (r = 0.52, p = 0.009; r = 0.504, p = 0.012; and r = 0.58, p = 0.003; respectively) and negative correlations with ALT levels (r = −0.42, p = 0.04; r = −0.49, p = 0.016; and r = −0.472, p = 0.02; respectively).
Conclusion
The results of the study suggest that TLR3 and TLR9 play a role in the pathogenesis of SLE, and have an impact on organ involvement in this disease. More studies concerning the biology and function of TLRs are required in larger patient cohorts, and may lead to development of a new class of drugs.
Zusammenfassung
Hintergrund
Der systemische Lupus erythematodes (SLE) stellt eine verbreitete und komplexe Erkrankung dar, die typischerweise mit chronischen generalisierten Entzündungsprozessen einhergeht, welche verschiedene Gewebe und Organe betreffen können.
Ziel
Ziel der vorliegenden Arbeit war, die Expression der Toll-like-Rezeptoren (TLR) 3 und 9 bei SLE-Patienten sowie ihren Zusammenhang mit klinischen Merkmalen, Krankheitsaktivität und Schädigungen zu untersuchen.
Patienten und Methoden
In die aktuelle Studie wurden 24 ägyptische SLE-Patientinnen und 15 abgestimmte Kontrollen aufgenommen. Die Krankheitsaktivität wurde anhand des SLE-Disease-Activity-Index (SLEDAI) und die Schädigungen mit dem Systemic-Lupus-International-Collaborating-Clinics(SLICC)-Index erfasst. Die Expression von TLR3 und TLR9 in B- (CD19-positiv) und T-Lymphozyten (CD3-positiv) wurde unter Einsatz der Durchflusszytometrie untersucht.
Ergebnisse
Das Patientenalter lag zwischen 17 und 42 Jahren (Mittel: 26,17 ± 5,78 Jahre). Es bestand ein signifikanter Unterschied zwischen Patienten und Kontrollen hinsichtlich der Expression von TLR3/CD3, TLR3/CD19, TLR9/CD3 bzw. TLR9/CD19 (p < 0,0001). Eine signifikante Korrelation bestand zwischen TLR3/CD3, TLR3/CD19 bzw. TLR9/CD19 und dem Serumkreatinin (r = 0,52; p = 0,009; r = 0,504; p = 0,012 bzw. r = 0,58; p = 0,003) und eine negative Korrelation mit den Werten für Alanintransaminase (ALT; r = −0,42; p = 0,04 bzw. r = −0,49; p = 0,016 bzw. r = −0,472; p = 0,02).
Schlussfolgerung
Den Ergebnisse der Studie zufolge spielen TLR3 und TLR9 eine Rolle in der Pathogenese des SLE und haben Einfluss auf die Organbeteiligung bei dieser Erkrankung. Weitere Studien zur Biologie und Funktion der TLR an größeren Patientenkohorten sind erforderlich und können möglicherweise zur Entwicklung einer neuen Klasse von Medikamenten führen.
Similar content being viewed by others
References
Hanly JG, Su L, Farewell V, Fritzler MJ (2010) Comparison between multiplex assays for autoantibody detection in systemic lupus erythematosus. J Immunol Methods 358(1–2):75–80
Hassan AB, Lundberg IE, Isenberg D, Wahren-Herlenius M (2002) Serial analysis of Ro/SSA and La/SSB antibody levels and correlation with clinical disease activity in patients with systemic lupus erythematosus. Scand J Rheumatol 31(3):133–139
Smith EL, Shmerling RH (1999) The American College of Rheumatology criteria for the classification of systemic lupus erythematosus: strengths, weaknesses, and opportunities for improvement. Lupus 8(8):586–595
Guggino G, Giardina AR, Ciccia F, Triolo G, Dieli F, Sireci G (2012) Are Toll-like receptors and decoy receptors involved in the immunopathogenesis of systemic lupus erythematosus and lupus-like syndromes? Clin Dev Immunol 2012:135932
Hurst J, von Landenberg P (2008) Toll-like receptors and autoimmunity. Autoimmun Rev 7(3):204–208
Lee BL, Barton GM (2014) Trafficking of endosomal toll-like receptors. Trends Cell Biol 24:360–369
Kim YM, Brinkmann MM, Paquet ME, Ploegh HL (2008) UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452:234–238
Komatsuda A, Wakui H, Iwamoto K, Ozawa M, Togashi M, Masai R et al (2008) Up-regulated expression of Toll-like receptors mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Exp Immunol 152:482–487
Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T et al (2010) TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature 465:937–941
Bijl M, Limburg PC, Kallenberg CG (2001) New insights into the pathogenesis of systemic lupus erythematosus (SLE): the role of apoptosis. Neth J Med 59:66–75
Lorenz HM, Herrmann M, Winkler T, Gaipl U, Kalden JR (2000) Role of apoptosis in autoimmunity. Apoptosis 5:443–449
Kaplan MJ (2004) Apoptosis in systemic lupus erythematosus. Clin Immunol 112:210–218
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783
Rahman AH, Eisenberg RA (2006) The role of toll-like receptors in systemic lupus erythematosus. Springer Semin Immunopathol 28:131–143
Thoma-Uszynski S, Stenger S, Ochoa Takeuchi OMT, Engele M, Sieling PA et al (2001) Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291:1544–1547
Blander JM, Medzhitov R (2006) Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440:808–812
Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR et al (2012) Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64(8):2677–2686
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum 35:630–640
Gladman DD, Urowitz MB, Goldsmith CH, Fortin P, Ginzler E, Gordon C et al (1997) The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 40:809–813
Wong CK, Wong PT, Tam LS, Li EK, Chen DP, Lam CW (2010) Activation profile of Toll-like receptors of peripheral blood lymphocytes in patients with systemic lupus erythematosus. Clin Exp Immunol 159(1):11–22
Guggino G, Giardina AR, Ciccia F, Triolo G, Dieli F, Sireci G (2012) Are Toll-like receptors and decoy receptors involved inthe immunopathogenesis of systemic lupus erythematosus and lupus-like syndromes? Clin Dev Immunol 2012:135932
Kulkarni O, Anders HJ (2008) Chemokines in lupus nephritis. Front Biosci 13:3312–3320
Lourenço EV, La Cava A (2009) Cytokines in systemic lupus erythematosus. Curr Mol Med 9:242–254
Fischer M, Ehlers M (2008) Toll-like receptors in autoimmunity. Ann NY Acad Sci 1143:21–34
Tsao JT, Hsieh SC, Chiang BL, Yu CL, Lin SC (2012) Altered IL-10 and TNF-α production in peripheral blood mononuclear cells of systemic lupus erythematosus patients after Toll-like receptor 2, 4, or 9 activation. Clin Exp Med 12(3):153–158
Klonowska-Szymczyk A, Wolska A, Robak T, Cebula-Obrzut B, Smolewski P, Robak E (2014) Expression of toll-like receptors 3, 7 and 9 in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Mediat Inflamm 2014:381418
Wu O, Chen GP, Chen H, Li XP, Xu JH, Zhao SS et al (2009) The expressions of Toll-like receptor 9 and T-bet in circulating B and T cells in newly diagnosed, untreated systemic lupus erythematosus and correlations with disease activity and laboratory data in a Chinese population. Immunobiology 214(5):392–402
Papadimitraki ED, Choulaki C, Koutala E, Bertsias G, Tsatsanis C, Gergianaki I et al (2006) Expansion of toll-like receptor 9-expressing B cells in active systemic lupus erythematosus: implications for the induction and maintenance of the autoimmune process. Arthritis Rheum 54(11):3601–3611
Patole PS, Gröne HJ, Segerer S, Ciubar R, Belemezova E, Henger A et al (2005) Viral double stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J Am Soc Nephrol 16:1326–1338
Migita K, Miyashita T, Maeda Y, Nakamura M, Yatsuhashi H, Kimura H et al (2007) Toll-like receptor expression in lupus peripheral blood mononuclear cells. J Rheumatol 34(3):493–500
Sieber J, Daridon C, Fleischer SJ, Fleischer V, Hiepe F, Alexander T et al (2014) Active systemic lupus erythematosus is associated with a reduced cytokine production by B cells in response to TLR9 stimulation. Arthritis Res Ther 16(6):477
Zorro S, Arias M, Riano F, Paris S, Ramirez LA, Uribe O et al (2009) Response to ODN-CpG by B Cells from patients with systemic lupus erythematosus correlates with disease activity. Lupus 18:718–726
Tsokos GC (2011) Systemic lupus erythematosus. N Engl J Med 365:2110–2121
Liew FY, Xu D, Brint EK, O’Neill LA (2005) Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5:446–458
Nickerson KM, Christensen SR, Cullen JL, Meng W, Luning PET, Shlomchik MJ (2013) TLR9 promotes tolerance by restricting survival of anergic anti-DNA B cells, yet is also required for their activation. J Immunol 190:1447–1456
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. S. Nasr, S. M. Fawzy, T. A. Gheita, and E. El-Khateeb state that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Rights and permissions
About this article
Cite this article
Nasr, A.S., Fawzy, S.M., Gheita, T.A. et al. Expression of Toll‑like receptors 3 and 9 in Egyptian systemic lupus erythematosus patients. Z Rheumatol 75, 502–507 (2016). https://doi.org/10.1007/s00393-015-0022-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-015-0022-x