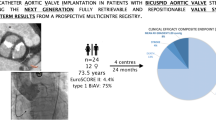
Abstract
Background
Patients undergoing transcatheter aortic valve implantation (TAVI) for bicuspid aortic stenosis (AS) frequently present with ascending aortic (AAo) dilatation which is left untreated. The objective of this study was to study the natural progression and underlying mechanisms of AAo dilatation after TAVI for bicuspid AS.
Methods
Patients with a native bicuspid AS and a baseline AAo maximum diameter > 40 mm treated by TAVI and in whom post-TAVI computed tomography (CT) scans beyond 1 year were available were included. AAo dilatation was deemed to be either continuous (≥ 2 mm increase) or stable (< 2 mm increase or decrease). Uni- and multivariate logistic regression analysis was utilized in order to identify factors associated with continuous AAo dilatation post-TAVI.
Results
A total of 61 patients with a mean AAo maximum diameter of 45.6 ± 3.9 mm at baseline were evaluated. At a median follow-up of 2.9 years, AAo dimensions remained stable in 85% of patients. Continuous AAo dilatation was observed in 15% of patients at a rate of 1.4 mm/year. Factors associated with continuous AAo dilatation were raphe length/annulus mean diameter ratio (OR 4.09, 95% CI [1.40–16.7], p = 0.022), TAV eccentricity at the leaflet outflow level (OR 2.11, 95%CI [1.12–4.53], p = 0.031) and maximum transprosthetic gradient (OR 1.30, 95%CI [0.99–1.73], p = 0.058).
Conclusions
Ascending aortic dilatation in patients undergoing TAVI for bicuspid AS remains stable in the majority of patients. Factors influencing TAV stent frame geometry and function were identified to be associated with continuous AAo dilatation after TAVI; this should be confirmed in future larger cohort studies.
Graphical Abstract

Progression of ascending aortic dilatation in bicuspid aortic stenosis patients who underwent TAVI. Among patients who underwent TAVI for bicuspid aortic valve stenosis, a high prevalence of ascending aortic (AAo) dilatation was observed, which remained stable in 85% of patients at follow-up. In 15% of patients, continuous AAo dilatation was noted and this was associated with an elevated trans-prosthetic maximum gradient, TAV eccentricity at the leaflet outflow level and an elevated ratio of the raphe length/aortic annulus mean diameter. AAo, ascending aorta dilatation; AS, aortic stenosis; CI, confidence interval; CT, computed tomography; max, maximum; OR, odds ratio; TAV, transcatheter aortic valve; TAVI, transcatheter aortic valve implantation; Ø, diameter. Some of the CMR images were used with permission from the copyright owner (Secinaro et al. in Eur Heart J Cardiovasc Imaging 00:1–8, 2021).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bicuspid aortic valve (BAV) disease is a common cardiac pathology frequently associated with an aortopathy [1, 2]. The development of BAV aortopathy can occur independent of valvular alterations and can lead to pronounced ascending aortic (AAo) dilatation, aneurysm formation and subsequent increased risk for acute aortic events [3,4,5,6]. Therefore, current guidelines recommend prophylactic aortic surgery in patients with BAV disease and a dilated aorta with or without valvular dysfunction [7,8,9].
Transcatheter aortic valve implantation (TAVI) can effectively treat severe symptomatic aortic stenosis (AS). However, in patients with severe bicuspid AS and significant aortopathy undergoing TAVI, the dilated aorta is left untreated. As TAVI expands to younger patients, an increased proportion of BAV disease with associated AAo dilatation is expected to be encountered. [10, 11] In these patients with longer life-expectancy, the causes and long-term consequences of leaving behind a dilated AAo demand attention. Unfortunately, data on this topic is scarce. [12,13,14,15]
In this study, we used pre- and post-TAVI computed tomography (CT) scans to evaluate the natural progression in AAo dilatation following TAVI in patients presenting with bicuspid aortic valve stenosis and baseline AAo dilatation.
Methods
Study population
Among 969 patients who underwent TAVI for bicuspid AS between 2008 and 2022 from four centres (Rigshospitalet, Denmark; AOU Policlinico “G. Rodolico-San Marco”, Italy; Bern University Hospital, Switzerland; West China Hospital, China), pre-TAVI CT was used to identify 464 patients with a dilated aorta, defined as a maximum AAo diameter ≥ 40 mm. [16] Of these, a high-quality post-implant cardiac CT obtained beyond one year was available in 61 patients, which were analyzed for this study (Fig. 1). All cardiac CT scans were electrocardiographically gated, contrast enhanced and thin-sliced (≤ 1.0 mm slice thickness). CT analysis was performed using systolic images, or in the case of motion artefact, the best quality diastolic images. Measurements were all performed and independently verified by physicians experienced in pre- and post-TAVI CT analysis, using 3Mensio software (Pie Medical Imaging, the Netherlands). Patients who underwent TAVI for degenerated surgical bioprosthesis or with a previous history of percutaneous or surgical aortic intervention were excluded. Ethical approval for the study was granted by the local ethical committees and written informed consent was obtained from all patients included.
Study population. The study population was derived from a cohort of patients who underwent TAVI for bicuspid aortic stenosis (AS) in whom pre-TAVI computed tomography (CT) identified a baseline ascending aortic diameter > 40 mm. Patients with post-implantation CT beyond 1 year after TAVI were included for analysis. AAo ascending aorta; AS aortic stenosis; CT computed tomography; max maximum; TAVI transcatheter aortic valve implantation; Ø diameter
Measurement of ascending aortic dilatation
Multi-planar reconstructions were created to measure the minimum, maximum and mean cross-sectional diameters of the sinus of Valsalva (SoV), sinotubular junction (STJ) and the AAo (defined as the tubular aorta arising above the STJ to the level of the pulmonary artery bifurcation) in a plane perpendicular to the central axis of the aorta. The location of the maximum diameter in the AAo was noted with reference to the height from the aortic annular plane to allow for comparison on post-implant CT-scans.
The above measurements were all repeated on the post-TAVI CT-scans. The amount of AAo dilatation post-TAVI was evaluated by calculating the difference in maximum AAo diameter between the pre- and post-TAVI CT. An increase in AAo diameter ≥ 2 mm was considered as continuous dilatation, whereas a decrease or < 2 mm increase in AAo diameter was considered as stable (Fig. 2). The rate of AAo dilatation was calculated as the difference in maximum AAo diameter divided by the time interval in years for each patient.
Progression of ascending aortic dilatation after TAVI. Individual patient-level data are presented for the absolute change (Δ) in the maximum and mean AAo diameter after TAVI; this for bicuspid AS patients treated with TAVI and having a baseline maximum AAo diameter > 40 mm. At follow-up, AAo diameters remained stable in the majority of patients. Continuous AAo dilatation after TAVI with a more than 2 mm increase in the maximum and mean AAo diameter was noted in 9 and 8 patients, respectively. AAo ascending aorta; CT computed tomography; max maximum; TAVI transcatheter aortic valve implantation; Ø diameter
Native and transcatheter aortic valve analysis
Pre-TAVI CT scans were used to determine the morphology of the bicuspid aortic valve, measure the raphe length and quantify the volume of calcification (mm3) within both the raphe and native aortic valve leaflets [17].
Post-TAVI CT scans were used to evaluate the TAV stent frame geometry. Cross-sectional maximum and minimum TAV stent frame diameters, perimeters and areas were measured at the level of the leaflet inflow and outflow. TAV expansion was calculated as:
TAV eccentricity was calculated using the following formula as previously described [18]:
The value of eccentricity varies between 0 and 1, with a smaller value (closer to 0) representing a more circular shape of the TAV stent frame. Both TAV expansion and eccentricity were measured at the leaflet inflow and outflow levels for each specific TAV.
The TAV performance and hemodynamics were assessed in all patients using transthoracic echocardiography (TTE) prior to discharge from the index hospitalization. The maximum and mean transprosthetic AV gradients and effective orifice area (EOA), calculated using the continuity equation, were obtained.
Statistical analysis
Categorical variables are expressed as numbers (percentages) and continuous variables as mean ± SD or median (interquartile range). The cohort was divided into two groups: stable AAo and continuous AAo dilatation. Differences between the groups were analyzed using the Chi-square and Student’s t-test, where appropriate. A stepwise uni- and multivariate logistic regression analysis was utilized in order to identify associated factors and independent predictors of continuous AAo dilatation. Clinical, cardiac CT, procedural and post-TAVI echocardiographic variables were included in this analysis. Variables which were associated with continuous AAo dilatation in the univariate model (defined as p < 0.1) were included in the multivariate model in order to identify independent predictors of continuous AAo dilatation. For a ratio variable, the odds ratio was expressed for each 0.1 increase in both uni- and multivariate analyses. For maximum transprosthetic AV gradient, the odds ratio was expressed for each 5 mmHg increase. In case of multicollinearity (e.g., raphe length and raphe length/annulus mean diameter ratio), only the variable with the highest statistical power was included in the multivariate analysis. All statistical analyses were performed using SPSS Statistics Software (IBM, NY, USA).
Results
Study population
For this study, a total of 61 patients who underwent TAVI for bicuspid AS with a maximum AAo diameter > 40 mm at baseline and who had a cardiac CT at 1–7 years (median 2.9 years) post-procedure could be included. Baseline clinical, CT, procedural and echocardiographic details are summarized in Table 1. The mean age of the study cohort was 73.6 ± 6.6 years, 31.1% were female and the mean surgical risk score was 2.6 ± 1.9%. None of the patients was known with Marfan syndrome or any other connective tissue disorder. Bicuspid aortic valve Sievers type 1 was the most common phenotype in 84% of all patients, with right-left cusp fusion being the predominant BAV type. TAVI was performed using a self-expanding TAV in 67%, a balloon-expandable TAV in 30% and a mechanical TAV in 3% of patients.
Ascending aortic dilatation
At baseline, the maximum AAo diameter was 45.6 ± 3.9 mm and the mean AAo diameter was 44.5 ± 3.8 mm in the overall study cohort. The STJ was also relatively wide with a mean diameter of 34.2 ± 4.1 mm (Table 1).
Over a median study period of 2.9 years (IQR 1.6–3.7 years), the maximum AAo diameter remained stable in 85.2% of patients, whilst continuous AAo dilatation was noted in 14.8% of patients (Fig. 2). A > 5 mm increase in the maximum AAo diameter was observed in three patients and the AAo dilated beyond 55 mm in two patients. Among the nine patients with continuous dilatation based on a maximum AAo Ø expansion ≥ 2 mm, there was calculated an expansion rate of 1.4 mm/year, with the fastest expansion rate being 3.4 mm/year. In contrast the overall expansion rate for the entire cohort was 0.2 mm/year.
TAV geometry and hemodynamics
At post-implant CT, TAV prostheses were found to be under-expanded and eccentric in shape across the entire cohort. Average TAV expansion at the leaflet inflow and outflow level was 0.65 ± 0.19 and 0.89 ± 0.34, respectively, whilst TAV eccentricity at the leaflet inflow and outflow level was 0.51 ± 0.16 and 0.42 ± 0.12, respectively.
At pre-discharge TTE, the mean and maximum trans-prosthetic valve gradient was 11.6 ± 6.9 mmHg and 21.9 ± 11.8 mmHg, respectively, with a calculated mean EOA of 1.8 ± 0.6 cm2 (Table 1).
Associated factors and predictors of AAo dilatation after TAVI
Multiple clinical, CT, procedural and echocardiographic variables were screened to identify variables associated with AAo dilatation after TAVI. Both statistical analysis using Chi-square and Student’s t test as well as univariate logistic regression analysis could identify the ratio of raphe length/aortic annulus mean Ø, TAV eccentricity at leaflet outflow level and maximum trans-prosthetic gradient as factors associated with continuous AAo dilatation. None of the clinical variables or baseline aortic dimensions were associated factors (Table 2).
In the multivariate analysis, the ratio of raphe length/aortic annulus mean Ø remained the strongest independent predictor for continuous AAo dilatation (OR 5.67, 95% CI [1.50–35.3], p = 0.026); TAV eccentricity (OR 2.20, 95% CI [1.00–6.65], p = 0.093) and maximum AV gradient (OR 1.11, 95% CI [0.74–1.64], p = 0.598) were less predictive variables (Table 3).
Discussion
In this unique dataset of pre- and post-implant CT scans from patients who underwent TAVI for bicuspid AS with baseline AAo dilatation, the key conclusions are as follows: (1) at a median follow-up of 2.9 years, AAo dimensions remained stable in 85% of patients, (2) continuous AAo dilatation at an average rate of 1.4 mm/year was observed in 15% of patients, and (3) the ratio of the raphe length/mean annulus diameter, TAV eccentricity and maximum AV gradient were associated with continuous AAo dilatation after TAVI (Graphical abstract).
Ascending aortic dilatation after valve intervention
Historical studies have consistently reported the relative stability in AAo dilatation following surgical aortic valve replacement (SAVR). [19,20,21,22] In the largest study of 448 patients with mixed bicuspid and tricuspid AV disease and varying degrees of aortopathy, surgical valve replacement only (sparing the dilated aorta) was associated with no significant increase in AAo dimensions at a mean follow-up of 7.5 ± 3.9 years. [19]
Similarly, prior TTE- and CT-based follow-up studies of patients undergoing TAVI for mixed bicuspid or tricuspid AV disease, reported relative stability in AAo dimensions at medium-term follow-up. [12,13,14,15] In a CT-based follow-up study (median follow-up of 1.7 years) of 208 tricuspid and bicuspid AV patients undergoing TAVI with baseline aortic dilatation (mean diameter 41 ± 5.1 mm), a mean increase in AAo diameter of 0.5 ± 1.0 mm and a mean dilatation rate of 0.3 ± 0.8 mm/year was observed. [13] Among 107 patients with moderate-to-severe AAo dilatation (mean diameter 48.6 ± 2.8 mm), the progression rate of AAo dilatation was 0.0 ( – 0.3–0.2) mm/year. [15] Our study complements these findings in a cohort of patients with exclusively bicuspid AS and baseline AAo dilatation. Although stability of AAo dilatation post-TAVI was observed in the majority (85%) of patients, 15% of patients had continuous AAo dilatation (> 2 mm) after TAVI at an average expansion rate of 1.4 mm/year.
Factors underlying continuous AAo dilatation post-TAVI
Progressive AAo dilatation in patients with bicuspid AV arises due to a complex interaction between the underlying genetics and hemodynamic factors [23,24,25]. Patients with bicuspid aortic valve disease exhibit abnormal helical and eccentric blood flow patterns, which result in increased aortic wall shear stress (WSS) [26, 27]. The downstream consequences of increased WSS are regional changes in gene expression, dysregulation of the extra-cellular matrix and increased elastic fiber degeneration leading to the characteristic asymmetric AAo dilatation observed in BAV disease [28].
The type, extent and location of these abnormal blood flow patterns can be influenced by the structure and phenotype of the bicuspid valve complex [26, 27] as well as the type of surgical or transcatheter aortic valve intervention [29,30,31]. Restoration of central laminar blood flow patterns following surgery is associated with improvements in WSS and subsequent stability in AAo dilatation [32,33,34]. In contrast, persistence of more eccentric helical blood flow patterns and resulting higher WSS values have been reported following surgical implantation of tissue bioprosthetic valve or after TAVI [29,30,31].
These changes in blood flow patterns following valvular intervention may explain why, in our study, certain variables were associated with continuous AAo dilatation after TAVI. A long and calcified raphe, resulting in an increased raphe/mean annulus diameter ratio, may cause tilting of the assembled TAV complex resulting in a non-central, eccentrically directed jet towards the aortic wall. Similarly, a non-circular eccentric TAV stent frame, at the level of the leaflet outflow, prevents a laminar aortic blood flow, creating abnormal non-laminar blood flow patterns, such as helical flows. These abnormal flow patterns can be further exacerbated by high flow velocities, as measured by higher trans-prosthetic gradients. Consequently, the non-centric, non-laminar and high velocity flow, generated by a sub-optimal structure and function of the implanted TAV, may worsen WSS on the aortic wall leading to continuous AAo dilatation. However, due to the relatively small cohort of patients, these findings should be interpreted as hypothesis-generating and further larger studies are required to determine a pathophysiological link between the assembled structure and function of the implanted TAV, aortic blood flow pattern abnormalities and WSS, and subsequent AAo dilatation.
Clinical implications
As TAVI expands to younger populations, an increasing proportion of patients with bicuspid AS and AAo dilatation will be encountered [10, 11]. In these patients with longer life-expectancies, the ability to predict, stabilise and monitor AAo dilatation becomes increasingly relevant. In this context, our study sheds new insights. First, baseline CT scans may be used to identify variables predictive of continuous AAo dilatation after TAVI, which may be instructive when trying to determine the optimal lifetime management strategy for younger patients with BAV disease and AAo dilatation [35]. Intra-procedurally, efforts to achieve a well-expanded circular TAV with low residual gradients may be important to ensure optimal aortic blood flow patterns to stabilise AAo dilatation. Finally, in patients with baseline AAo dilatation and unfavourable BAV anatomy or TAV implantation, a close surveillance of the AAo should be considered. However, further studies are warranted to determine the optimal time, duration and imaging modality necessary for monitoring.
Limitations
Our study is based on a relatively small cohort of patients; therefore, the conclusions should be considered hypothesis-generating and require validation in a larger patient cohort. Due to the small sample size of patients with continuous AAo dilatation, potential other clinical, baseline CT or TAV-related factors associated with continuous AAo dilatation cannot be excluded. Also, systematic genetic testing was not available. The median duration of follow-up was 2.9 years; the longer term AAo diameter stability and natural progression of AAo dilatation up to 5 to 10 years remains unknown. Although no patients with continuous AAo dilatation experienced an adverse aortic event, a selection bias cannot be excluded, as only patients in whom post-TAVI CT scans beyond one year were available were included. Finally, this study only included patients with bicuspid AS; hence, these study findings cannot be translated to patients with bicuspid aortic regurgitation or tricuspid AV disease.
Conclusions
Ascending aortic dilatation remains stable in the majority of patients undergoing TAVI for bicuspid AS. In 15% of patients, progression in aortic dilatation is observed, which seems to be related to the structure and function of the implanted TAV. The ratio of the raphe length to aortic annulus mean diameter was the strongest predictor for continuous ascending aortic dilatation after TAVI.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AAo:
-
Ascending aorta
- AS:
-
Aortic stenosis
- AV:
-
Aortic valve
- BAV:
-
Bicuspid aortic valve
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- SAVR:
-
Surgical aortic valve replacement
- SoV:
-
Sinus of Valsalva
- STJ:
-
Sinotubular junction
- TAV:
-
Transcatheter aortic valve
- TAVI:
-
Transcatheter aortic valve implantation
- WSS:
-
Wall shear stress
References
Michelena HI, Khanna AD, Mahoney D et al (2011) Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 306(10):1104–1112. https://doi.org/10.1001/jama.2011.1286
Verma S, Siu SC (2014) Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 370(20):1920–1929. https://doi.org/10.1056/nejmra1207059
Tzemos N, Therrien J, Yip J et al (2008) Outcomes in adults with bicuspid aortic valves. JAMA 300(11):1317–1325. https://doi.org/10.1001/jama.300.11.1317
Oladokun D, Patterson BO, Sobocinski J et al (2016) Systematic review of the growth rates and influencing factors in thoracic aortic aneurysms. Eur J Vasc Endovasc Surg. https://doi.org/10.1016/j.ejvs.2016.01.017
Agnese V, Pasta S, Michelena HI et al (2019) Patterns of ascending aortic dilatation and predictors of surgical replacement of the aorta: a comparison of bicuspid and tricuspid aortic valve patients over eight years of follow-up. J Mol Cell Cardiol 135:31–39. https://doi.org/10.1016/j.yjmcc.2019.07.010
Yang L-T, Ye Z, Wajih UM et al (2023) Bicuspid aortic valve: long-term morbidity and mortality. Eur Heart J 44(43):4549–4562. https://doi.org/10.1093/eurheartj/ehad477
Otto CM, Nishimura RA, Bonow RO et al (2020) ACC/aha guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 2021:E72-227. https://doi.org/10.1161/CIR.0000000000000923
Vahanian A, Beyersdorf F, Praz F et al (2022) 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 43(7):561–632. https://doi.org/10.1093/eurheartj/ehab395
Czerny M, Grabenwöger M, Berger T et al (2024) EACTS/STS guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur J Cardio-Thoracic Surg. https://doi.org/10.1093/ejcts/ezad426
Ochiai T, Yoon SH, Sharma R et al (2020) Prevalence and prognostic impact of ascending aortic dilatation in patients undergoing TAVI. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2019.07.025
Jia Y, Tirado-Conte G, Montarello N et al (2023) Prognostic Impact of ascending aortic dilatation in bicuspid TAVI patients. JACC Cardiovasc Interv. https://doi.org/10.1016/j.jcin.2023.09.015
Rylski B, Szeto WY, Bavaria JE et al (2014) Transcatheter aortic valve implantation in patients with ascending aortic dilatation: safety of the procedure and mid-term follow-up. Eur J Cardio-Thoracic Surg 46(2):228–233. https://doi.org/10.1093/ejcts/ezt594
He YX, Fan JQ, Zhu QF et al (2019) Ascending aortic dilatation rate after transcatheter aortic valve replacement in patients with bicuspid and tricuspid aortic stenosis: a multidetector computed tomography follow-up study. World J Emerg Med 10(4):197–204. https://doi.org/10.5847/wjem.j.1920-8642.2019.04.001
Jung JH, Kim HK, Park JB et al (2021) Progression of ascending aortopathy may not occur after transcatheter aortic valve replacement in severe bicuspid aortic stenosis. Korean J Intern Med 36(2):332–341. https://doi.org/10.3904/KJIM.2019.089
Feng D, Zhao J, Niu G et al (2024) Outcomes for patients undergoing transcatheter aortic valve replacement with ascending aorta dilation. Int J Cardiol. https://doi.org/10.1016/j.ijcard.2024.131948
Kerneis C, Pasi N, Arangalage D et al (2018) Ascending aorta dilatation rates in patients with tricuspid and bicuspid aortic stenosis: the COFRASA/GENERAC study. Eur Heart J Cardiovasc Imaging 19(7):792–799. https://doi.org/10.1093/ehjci/jex176
Sievers HH, Schmidtke C (2007) A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 133(5):1226–1233. https://doi.org/10.1016/j.jtcvs.2007.01.039
Fukui M, Bapat VN, Garcia S et al (2022) Deformation of transcatheter aortic valve prostheses: implications for hypoattenuating leaflet thickening and clinical outcomes. Circulation 146(6):480–493. https://doi.org/10.1161/CIRCULATIONAHA.121.058339
Charitos EI, Stierle U, Petersen M et al (2014) The fate of the bicuspid valve aortopathy after aortic valve replacement. Eur J Cardio-Thoracic Surg. https://doi.org/10.1093/ejcts/ezt666
Girdauskas E, Rouman M, Disha K et al (2016) The fate of mild-to-moderate proximal aortic dilatation after isolated aortic valve replacement for bicuspid aortic valve stenosis: a magnetic resonance imaging follow-up study. Eur J Cardio-Thoracic Surg 49(4):e80–e87. https://doi.org/10.1093/ejcts/ezv472
Girdauskas E, Disha K, Borger MA, Kuntze T (2014) Long-term prognosis of ascending aortic aneurysm after aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Thorac Cardiovasc Surg 147(1):276–282. https://doi.org/10.1016/j.jtcvs.2012.11.004
Disha K, Rouman M, Secknus MA, Kuntze T, Girdauskas E (2016) Are normal-sized ascending aortas at risk of late aortic events after aortic valve replacement for bicuspid aortic valve disease? Interact Cardiovasc Thorac Surg 22(4):465–471. https://doi.org/10.1093/icvts/ivv387
Hope MD, Hope TA, Crook SES et al (2011) 4D flow CMR in assessment of valve-related ascending aortic disease. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2011.05.004
Barker AJ, Markl M, Bürk J et al (2012) Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 5(4):457–466. https://doi.org/10.1161/CIRCIMAGING.112.973370
Meierhofer C, Schneider EP, Lyko C et al (2013) Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: a prospective study. Eur Heart J Cardiovasc Imaging 14(8):797–804. https://doi.org/10.1093/ehjci/jes273
Bissell MM, Hess AT, Biasiolli L et al (2013) Aortic dilation in bicuspid aortic valve disease: flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging 6(4):499–507. https://doi.org/10.1161/CIRCIMAGING.113.000528
Stephens EH, Hope TA, Kari FA et al (2015) Greater asymmetric wall shear stress in Sievers’ type 1/LR compared with 0/LAT bicuspid aortic valves after valve-sparing aortic root replacement. J Thorac Cardiovasc Surg 150(1):59–68. https://doi.org/10.1016/j.jtcvs.2015.04.020
Guzzardi DG, Barker AJ, Van Ooij P et al (2015) Valve-related hemodynamics mediate human bicuspid aortopathy: insights from wall shear stress mapping. J Am Coll Cardiol 66(8):892–900. https://doi.org/10.1016/j.jacc.2015.06.1310
Bissell MM, Loudon M, Hess AT et al (2018) Differential flow improvements after valve replacements in bicuspid aortic valve disease: a cardiovascular magnetic resonance assessment. J Cardiovasc Magn Reson. https://doi.org/10.1186/s12968-018-0431-5
Trauzeddel RF, Löbe U, Barker AJ et al (2016) Blood flow characteristics in the ascending aorta after TAVI compared to surgical aortic valve replacement. Int J Cardiovasc Imaging 32(3):461–467. https://doi.org/10.1007/s10554-015-0792-x
Farag ES, Vendrik J, van Ooij P et al (2019) Transcatheter aortic valve replacement alters ascending aortic blood flow and wall shear stress patterns: a 4D flow MRI comparison with age-matched, elderly controls. Eur Radiol 29(3):1444–1451. https://doi.org/10.1007/s00330-018-5672-z
Kamada H, Ota H, Nakamura M et al (2020) Perioperative hemodynamic changes in the thoracic aorta in patients with aortic valve stenosis: a prospective serial 4D-flow MRI study. Semin Thorac Cardiovasc Surg 32(1):25–34. https://doi.org/10.1053/j.semtcvs.2019.07.006
Bollache E, Fedak PWM, Van Ooij P et al (2018) Perioperative evaluation of regional aortic wall shear stress patterns in patients undergoing aortic valve and/or proximal thoracic aortic replacement. J Thorac Cardiovasc Surg 155(6):2277–22862. https://doi.org/10.1016/j.jtcvs.2017.11.007
Cave DGW, Panayiotou H, Bissell MM (2021) Hemodynamic profiles before and after surgery in bicuspid aortic valve disease—a systematic review of the literature. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2021.629227
Windecker S, Okuno T, Unbehaun A, Mack M, Kapadia S, Falk V (2022) Which patients with aortic stenosis should be referred to surgery rather than transcatheter aortic valve implantation? Eur Heart J. https://doi.org/10.1093/eurheartj/ehac105
Secinaro A, Milano EG, Ciancarella et al (2021) Blood flow characteristics after aortic valve neocuspidization in paediatric patients: a comparison with the Ross procedure. Eur Heart J Cardiovasc Imaging 00:1–8. https://doi.org/10.1093/ehjci/jeab009
Funding
Open access funding provided by National Hospital. None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Arif A Khokhar received speaker fees from Boston Scientific and consultancy fees from Machnet Medical; Marco Barbanti is consultant for Edwards Lifesciences, Medtronic Inc., and Boston Scientific; Corrado Tamburino received speaker honoraria from Medtronic; Thomas Pilgrim reports research, travel or educational grants to the institution without personal remuneration from Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic and ATSens, and speaker fees and consultancy fees to the institution from Biotronik, Boston Scientific, Edwards Lifesciences, Abbott, Medtronic, Biosensors, and Highlife; Mao Chen is a consultant/proctor for Peijia Medical and Venus MedTech; Ole De Backer received consulting fees and institutional research grants from Abbott, Boston Scientific and Medtronic.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, Y., Khokhar, A.A., Pilgrim, T. et al. Incidence and predictors of continued ascending aortic dilatation after TAVI in patients with bicuspid aortic stenosis. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02545-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02545-9