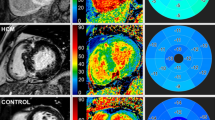
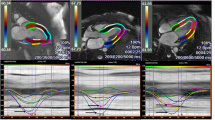
Abstract
Aim
To evaluate the ability of fast strain-encoded (SENC) cardiac magnetic resonance (CMR) derived myocardial strain and native T1 mapping to discriminate between hypertrophic cardiomyopathy (HCM) and cardiac amyloidosis.
Methods
Ninety nine patients (57 with hypertrophic cardiomyopathy and 42 with cardiac amyloidosis) were systematically analysed. LV-ejection fraction, LV-mass index, septal wall thickness and native T1 mapping values were assessed. In addition, global circumferential and longitudinal strain and segmental circumferential and longitudinal strain in basal, mid-ventricular, and apical segments were calculated. A ratio was built by dividing native T1 values by basal segmental strain (T1-to-basal segmental strain ratio).
Results
Myocardial strain was equally distributed in apical and basal segments in HCM patients, whereas an apical sparing with less impaired apical strain was noticed in cardiac amyloidosis (apical-to-basal-ratio of 1.01 ± 0.23 versus 1.20 ± 0.28, p < 0.001). T1 values were significantly higher in amyloidosis compared to HCM patients (1170.7 ± 66.4 ms versus 1078.3 ± 57.4ms, p < 0.001). The T1-to-basal segmental strain ratio exhibited high accuracy for the differentiation between the two clinical entities (Sensitivity = 85%, Specificity = 77%, AUC = 0.90, 95% CI = 0.81–0.95, p < 0.001). Multivariable analysis showed that age and the T1-to-basal-strain-ratio were the most robust factors for the differentiation between HCM and cardiac amyloidosis.
Conclusion
The T1-to-basal-segmental strain ratio, combining information from segmental circumferential and longitudinal strain and native T1 mapping aids the differentiation between HCM and cardiac amyloidosis with high accuracy and within a fast CMR protocol, obviating the need for contrast agent administration.
Graphical abstract



Similar content being viewed by others
Data availability
The dataset used and/or analysed is available from the corresponding author upon reasonable request.
Abbreviations
- LVH:
-
Left-ventricular-hypertrophy
- HCM:
-
Hypertrophic cardiomyopathy
- LV:
-
Left ventricle
- CMR:
-
Cardiovascular magnetic resonance
- Fast-SENC:
-
Fast strain-encoded sequence
- GLS:
-
Global longitudinal strain
- GCS:
-
Global circumferential strain
- AUC:
-
Area under the curve
- BSA:
-
Body surface area
- BMI:
-
Body-mass-index
- LVEDV:
-
Left-ventricular end-diastolic volume
- LVESV:
-
Left-ventricular end-systolic volume
- IVS:
-
Interventricular septum
References
McKenna WJ, Maron BJ, Thiene G (2017) Classification, Epidemiology, and Global Burden of Cardiomyopathies. Circ Res 121(7):722–730
Korosoglou G, Giusca S, Andre F et al (2021) Diagnostic work-up of cardiac amyloidosis using cardiovascular imaging: current standards and practical algorithms. Vasc Health Risk Manag 17:661–673
Glenner GG, Terry W, Harada M, Isersky C, Page D (1971) Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science 172(3988):1150–1151
Maurizi N, Rella V, Fumagalli C et al (2020) Prevalence of cardiac amyloidosis among adult patients referred to tertiary centres with an initial diagnosis of hypertrophic cardiomyopathy. Int J Cardiol 300:191–195
Ney S, Ihle P, Ruhnke T et al (2023) Epidemiology of cardiac amyloidosis in Germany: a retrospective analysis from 2009 to 2018. Clin Res Cardiol 112(3):401–408
Chamling B, Bietenbeck M, Korthals D et al (2023) Therapeutic value of tafamidis in patients with wild-type transthyretin amyloidosis (ATTRwt) with cardiomyopathy based on cardiovascular magnetic resonance (CMR) imaging. Clin Res Cardiol 112(3):353–362
Puntmann VO, Peker E, Chandrashekhar Y, Nagel E (2016) T1 Mapping in characterizing myocardial disease: a comprehensive review. Circ Res 119(2):277–299
Korosoglou G, Giusca S, Gitsioudis G, Erbel C, Katus HA (2014) Cardiac magnetic resonance and computed tomography angiography for clinical imaging of stable coronary artery disease. Diagnostic classification and risk stratification. Front Physiol 5: 291.
Giusca S, Steen H, Montenbruck M et al (2021) Multi-parametric assessment of left ventricular hypertrophy using late gadolinium enhancement, T1 mapping and strain-encoded cardiovascular magnetic resonance. J Cardiovasc Magn Reson 23(1):92
Tang W, McDonald SP, Hawley CM et al (2013) End-stage renal failure due to amyloidosis: outcomes in 490 ANZDATA registry cases. Nephrol Dial Transplant 28(2):455–461
Phelan D, Collier P, Thavendiranathan P et al (2012) Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 98(19):1442–1448
Liu D, Hu K, Niemann M et al (2013) Effect of combined systolic and diastolic functional parameter assessment for differentiation of cardiac amyloidosis from other causes of concentric left ventricular hypertrophy. Circ Cardiovasc Imaging 6(6):1066–1072
Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS (2014) Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol 64(1):83–99
Gertz MA, Comenzo R, Falk RH et al (2005) Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol 79(4):319–328
Prakken NH, Velthuis BK, Teske AJ, Mosterd A, Mali WP, Cramer MJ (2010) Cardiac MRI reference values for athletes and nonathletes corrected for body surface area, training hours/week and sex. Eur J Cardiovasc Prev Rehabil 17(2):198–203
Yilmaz A, Bauersachs J, Bengel F et al (2021) Diagnosis and treatment of cardiac amyloidosis: position statement of the German Cardiac Society (DGK). Clin Res Cardiol 110(4):479–506
Messroghli DR, Moon JC, Ferreira VM et al (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the society for cardiovascular magnetic resonance (SCMR) endorsed by the European association for cardiovascular imaging (EACVI). J Cardiovasc Magn Reson 19(1):75
Cardim N, Galderisi M, Edvardsen T et al (2015) Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging 16(3):280
Giusca S, Korosoglou G, Zieschang V et al (2018) Reproducibility study on myocardial strain assessment using fast-SENC cardiac magnetic resonance imaging. Sci Rep 8(1):14100
Korosoglou G, Giusca S, Hofmann NP et al (2019) Strain-encoded magnetic resonance: a method for the assessment of myocardial deformation. ESC Heart Fail 6(4):584–602
Steen H, Giusca S, Montenbruck M et al (2021) Left and right ventricular strain using fast strain-encoded cardiovascular magnetic resonance for the diagnostic classification of patients with chronic non-ischemic heart failure due to dilated, hypertrophic cardiomyopathy or cardiac amyloidosis. J Cardiovasc Magn Reson 23(1):45
Korosoglou G, Giusca S, Montenbruck M et al (2021) Fast Strain-Encoded Cardiac Magnetic Resonance for Diagnostic Classification and Risk Stratification of Heart Failure Patients. JACC Cardiovasc Imaging 14(6):1177–1188
Cerqueira MD, Weissman NJ, Dilsizian V, et al. (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation 105(4): 539–42.
Williams LK, Forero JF, Popovic ZB et al (2017) Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J Cardiovasc Magn Reson 19(1):61
Bravo PE, Fujikura K, Kijewski MF et al (2019) Relative apical sparing of myocardial longitudinal strain is explained by regional differences in total amyloid mass rather than the proportion of amyloid deposits. JACC Cardiovasc Imaging 12(7 Pt 1):1165–1173
Dass S, Suttie JJ, Piechnik SK et al (2012) Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging 5(6):726–733
Hosch W, Bock M, Libicher M et al (2007) MR-relaxometry of myocardial tissue: significant elevation of T1 and T2 relaxation times in cardiac amyloidosis. Invest Radiol 42(9):636–642
Fontana M, Banypersad SM, Treibel TA et al (2014) Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging 7(2):157–165
Lavall D, Vosshage NH, Gessner R et al (2023) Native T1 mapping for the diagnosis of cardiac amyloidosis in patients with left ventricular hypertrophy. Clin Res Cardiol 112(3):334–342
Karamitsos TD, Piechnik SK, Banypersad SM et al (2013) Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 6(4):488–497
Wang TKM, Brizneda MV, Kwon DH et al (2021) Reference ranges, diagnostic and prognostic utility of native T1 mapping and extracellular volume for cardiac amyloidosis: a meta-analysis. J Magn Reson Imaging 53(5):1458–1468
Pan JA, Kerwin MJ, Salerno M (2020) Native T1 mapping, extracellular volume mapping, and late gadolinium enhancement in cardiac amyloidosis: a meta-analysis. JACC Cardiovasc Imaging 13(6):1299–1310
Vo HQ, Marwick TH, Negishi K (2020) Pooled summary of native T1 value and extracellular volume with MOLLI variant sequences in normal subjects and patients with cardiovascular disease. Int J Cardiovasc Imaging 36(2):325–336
Lehman SJ, Crocini C, Leinwand LA (2022) Targeting the sarcomere in inherited cardiomyopathies. Nat Rev Cardiol 19(6):353–363
de Boer RA, Heymans S, Backs J, et al. (2022) Targeted therapies in genetic dilated and hypertrophic cardiomyopathies: from molecular mechanisms to therapeutic targets. A position paper from the Heart Failure Association (HFA) and the Working Group on Myocardial Function of the European Society of Cardiology (ESC). Eur J Heart Fail 24(3): 406–20.
Maurer MS, Schwartz JH, Gundapaneni B et al (2018) Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 379(11):1007–1016
Antonopoulos AS, Panagiotopoulos I, Kouroutzoglou A et al (2022) Prevalence and clinical outcomes of transthyretin amyloidosis: a systematic review and meta-analysis. Eur J Heart Fail 24(9):1677–1696
Bianchi G, Zhang Y, Comenzo RL (2021) AL amyloidosis: current chemotherapy and immune therapy treatment strategies: JACC: CardioOncology state-of-the-art review. JACC CardioOncol 3(4):467–487
Martens P, Hanna M, Valent J, Estep JD, Tang WHW (2023) Supra-normal left ventricular ejection fraction in cardiac amyloidosis. Clin Res Cardiol 112(3):441–443
Wheeler MT, Olivotto I, Elliott PM, et al. Effects of Mavacamten on Measures of Cardiopulmonary Exercise Testing Beyond Peak Oxygen Consumption: A Secondary Analysis of the EXPLORER-HCM Randomized Trial. JAMA Cardiol 2023.
Korthals D, Chatzantonis G, Bietenbeck M, Meier C, Stalling P, Yilmaz A (2021) CMR-based T1-mapping offers superior diagnostic value compared to longitudinal strain-based assessment of relative apical sparing in cardiac amyloidosis. Sci Rep 11(1):15521
Korosoglou G, Giusca S (2020) Strain for stress testing: Is it a featuring or supporting role in cardiac magnetic resonance? JACC Cardiovasc Imaging 13(1 Pt 1):66–68
Yue X, Yang L, Wang R et al (2022) The diagnostic value of multiparameter cardiovascular magnetic resonance for early detection of light-chain amyloidosis from hypertrophic cardiomyopathy patients. Front Cardiovasc Med 9:1017097
Korosoglou G, Ochs M (2023) Spotlight on myocardial deformation in hypertrophic cardiomyopathy: Putting the puzzle together? JACC Cardiovasc Imaging 16(4):492–494
Amr A, Koelemen J, Reich C, et al. (2023) Improving sudden cardiac death risk stratification in hypertrophic cardiomyopathy using established clinical variables and genetic information. Clin Res Cardiol.
Cheng S, Larson MG, McCabe EL et al (2013) Age- and sex-based reference limits and clinical correlates of myocardial strain and synchrony: the Framingham Heart Study. Circ Cardiovasc Imaging 6(5):692–699
Acknowledgements
We thank Birgit Hoerig, Kirsten Falk, Ute Pfeiffer and Silke Morgenstern for their excellent technical assistance with the acquisitions of all CMR scans.
Funding
The work had no external or internal funding.
Author information
Authors and Affiliations
Contributions
HS and GK designed the study, performed the analysis, and wrote the manuscript. All other authors performed the acquisitions and data analysis, reviewed the manuscript, and provided important intellectual input.
Corresponding author
Ethics declarations
Conflict of interests
HS, GK and SK received research grants from Myocardial Solutions. SK and HS own stock options of Myocardial Solutions. All other authors declare that they have no competing interests regarding this manuscript.
Ethics approval and consent to participate
The study was approved by Ethics Committee of the University of Hamburg (PV7193). All patients gave written informed consent.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Steen, H., Montenbruck, M., Kallifatidis, A. et al. Multi-parametric non-contrast cardiac magnetic resonance for the differentiation between cardiac amyloidosis and hypertrophic cardiomyopathy. Clin Res Cardiol 113, 469–480 (2024). https://doi.org/10.1007/s00392-023-02348-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02348-4