Abstract
Background
The impact of postero-anterior and medio-lateral mitral valve (MV) tethering patterns on outcomes in patients undergoing transcatheter edge-to-edge repair (M-TEER) for secondary mitral regurgitation (SMR) is unknown.
Methods
The ratio of the posterior to anterior MV leaflet angle (PLA/ALA) in MV segment 2 was defined as postero-anterior tethering asymmetry. Medio-lateral tethering asymmetry was assessed as the ratio of the medial (segment 3) to lateral (segment 1) MV tenting area. We used receiver-operating characteristics and a Cox regression model to identify cut-off values of asymmetric anteroposterior and medio-lateral tethering for prediction of 2 year all-cause mortality after TMVR.
Results
Among 178 SMR patients, postero-anterior tethering was asymmetric in 67 patients (37.9%, PLA/ALA ratio > 1.54). Asymmetric medio-lateral tethering (tenting area ratio > 1.49) was observed in 49 patients (27.5%). M-TEER reduced MR to ≤ 2 + in 92.1% of patients; MR reduction was less effective in the presence of asymmetric postero-anterior tethering (p = 0.02). A multivariable Cox regression model identified both types of asymmetric MV tethering to be associated with increased all-cause 2-year mortality (postero-anterior tethering asymmetry: HR = 2.77, CI 1.43–5.38; medio-lateral tethering asymmetry: HR = 2.90, CI 1.54–5.45; p < 0.01).
Conclusions
Asymmetric postero-anterior and medio-lateral MV tethering patterns are associated with increased 2-year mortality in patients undergoing M-TEER for SMR. A detailed echocardiographic analysis of MV anatomy may help to identify patients who profit most from M-TEER.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe secondary mitral regurgitation (SMR) has a poor prognosis and causes substantial morbidity in patients with heart failure and reduced ejection fraction (HFrEF) [1]. Therefore, mitral transcatheter valve edge-to-edge repair (M-TEER) is a guideline-recommended therapy in symptomatic high-risk patients with severe SMR after application of guideline-directed medical treatment and, if indicated, cardiac resynchronization therapy [2]. Two recent randomized-controlled trials (COAPT and MITRA-FR) showed conflicting results regarding hospitalization for heart failure and mortality [3, 4]. The results of both randomized studies and other registries stress the importance of meticulous preprocedural patient selection for M-TEER to achieve best possible results and long-lasting benefit [5, 6].
In HFrEF patients, left-ventricular ejection fraction (LV-EF) progressively deteriorates and left heart geometry alters due to ventricular, atrial, and annular dilation [7, 8]. Ventricular remodelling particularly affects the geometric configuration of the subvalvular mitral apparatus including papillary muscles, as the latter become displaced [9,10,11]. This leads to restriction of MV leaflets with consecutive MR defined as type IIIb in the Carpentier classification. The underlying pathology resulting in SMR is heterogeneous with ischemic cardiomyopathies resulting in ischemic SMR, dilated cardiomyopathies resulting in non-ischemic SMR, and finally atrial fibrillation and heart failure with preserved ejection fraction resulting in atrial SMR (ASMR) [12,13,14]. Thus, atrioventricular remodelling itself is a rather heterogeneous process and may lead to different patterns of MV leaflet tethering. In the current literature, the term asymmetric MV leaflet tethering is applied heterogeneously [15,16,17]. Some authors use it to describe a disproportionate restriction of the posterior, compared to the anterior MV leaflet motion [16]. Other authors use this term to describe a more severe tethering in medial segment 3 (S3) of the MV, compared to central S2 and lateral S1 as represented by tenting area and volume in the respective segments. Medio-lateral tethering asymmetry is believed to be a consequence of inferoposterior myocardial infarction (MI) with subsequent apical displacement of the medial papillary muscle [15]. Data about the impact of both postero-anterior and medio-lateral asymmetric MV tethering patterns on outcomes after M-TEER for severe SMR are absent. Only restricted posterior MV leaflet tethering has been reported to predict persistence or recurrence of MR severity > 2 + up to 12 months after intervention [18].
In patients undergoing MV surgery for SMR, asymmetric leaflet tethering patterns have been shown to be associated with increased rates of residual MR and worse long-term outcome [19, 20]. Since M-TEER is now the interventional therapeutic option of choice in SMR [2], the aim of this study was to evaluate the prognostic impact of asymmetric postero-anterior and medio-lateral leaflet tethering in M-TEER-treated SMR patients regarding procedural MR reduction, symptomatic improvement, and 2-year all-cause mortality.
Methods
Patient selection and treatment process
We included consecutive patients with moderate-to-severe (3+) or severe (4+) SMR undergoing M-TEER between July 2013 and March 2019 using MitraClip NT, NTR or XTR (Abbott, Santa Clara, California, USA) at our center. Due to significant changes of MV leaflet anatomy, patients with prior surgical or transcatheter mitral valve repair have been excluded from the analysis, as well as interventions being performed as bridge to heart transplantation or interventions with concomitant tricuspid transcatheter valve repair. Furthermore, patients without a detailed preprocedural echocardiographic assessment of the MV pathology were excluded from this study. After transthoracic (TTE) and transoesophageal echocardiographic (TEE) assessment, all patients were discussed by an interdisciplinary heart team and deemed at high or prohibitive surgical risk. Follow-up included survival status and New York Heart Association (NYHA) function class. Patients unable to attend follow-up examinations at our center were interviewed per telephone or seen by their local practitioners.
Echocardiography and endpoints
Echocardiographic evaluation of the cardiac chambers and MV was performed according to the recommendations of the European Association of Cardiovascular Imaging (EACVI) [21]. Detailed MV anatomy was assessed retrospectively by TEE image analysis. Papillary muscle distance and medio-lateral MV annular diameter were derived from a mid-oesophageal two-chamber view. Two-dimensional cross-sectional long-axis views were used for measurement of the following MV parameters in each MV segment: anterior (ALA) and posterior (PLA) leaflet angles were defined as angles between the mitral annular plane and tangent on the root of the anterior or posterior MV leaflet (Supplementary Fig. 1). Tenting height was measured as the distance between perpendicular lines through mitral annular plane reaching to the most ventricular located coaptation point of the MV leaflets. In the same frame, tenting area and postero-anterior MV annular diameter were assessed as shown in Supplementary Fig. 1. All anatomic MV measurements were performed for each MV segment in end-systole.
Effective regurgitant orifice area (EROA) and regurgitant volume (RegVol) were measured by the proximal isovelocity surface area method. The LV sphericity index was defined as ratio of LV length and width in an end-diastolic apical four-chamber view. LV width was measured as the broadest diameter of the LV in an apical four-chamber view. End-diastolic (LV-EDV) and end-systolic left-ventricular volume (LV-ESV) were measured using apical two- and four-chamber view according to Simpson’s biplane summation of disc method. Left-ventricular end-diastolic and systolic diameters, as well as MR vena contracta (MRVC) were obtained in a parasternal long-axis view. Systolic pulmonary artery pressure was estimated by addition of maximum systolic tricuspid valve pressure gradient with estimated right atrial pressure derived from inferior vena cava width. Grading of SMR severity was performed using a comprehensive approach integrating EROA, RegVol, MRVC, and jet morphology based on current guidelines [22, 23]. IntelliSpace Cardiovascular (Version 1.2, Philips Medical Systems, Nederland B.V.) was used for all echocardiographic analyses.
Two-year all-cause mortality was defined as primary endpoint. Postprocedural MR severity was assessed at the end of M-TEER procedure. Symptomatic improvement was evaluated using NYHA functional class.
Definition of postero-anterior and medio-lateral tethering
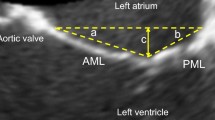
Postero-anterior MV leaflet tethering asymmetry was defined as the PLA/ALA ratio in MV segment 2 (Fig. 1A). Medio-lateral MV leaflet tethering asymmetry was assessed as the ratio of the MV tenting areas between MV segments 3 and 1 (Fig. 1B).
Symmetric and asymmetric postero-anterior and medio-lateral MV leaflet tethering. A Transoesophageal echocardiography of a patient with asymmetric postero-anterior tethering (left) and a patient with symmetric postero-anterior leaflet tethering (right). B Transoesophageal echocardiography of a patient with asymmetric medio-lateral leaflet tethering (top) and a patient with symmetric medio-lateral leaflet tethering (bottom). MV mitral valve; S segment
Statistical analysis
Normality of data was assessed using Kolmogorov–Smirnov and Shapiro–Wilk test. Mann–Whitney U or Pearson’s Chi-square test were used for inter-group comparison, as appropriate. The development of NYHA functional class and MR severity after M-TEER for each patient was analysed using the Wilcoxon test. We used receiver-operating characteristics (ROC) analysis and Youden’s J to identify the best discriminatory cut-off values for postero-anterior and medio-lateral asymmetric leaflet tethering in terms of 2-year all-cause mortality and residual postprocedural MR. Multivariable cox analysis and logistic regression included all parameters presenting with p < 0.05 in univariable statistics. The relationship of tethering symmetry and logarithmic hazard ratio was visualized using spline curves. Kaplan–Meier curves were used for showing survival after M-TEER. The difference of impact of tethering asymmetry on survival was calculated using the log-rank test. Results are displayed as hazard ratio (HR) with 95% confidence interval (CI) and p value. A p value of < 0.05 was defined as statistically significant. Statistical analyses were performed with SPSS (version 25, IBM, USA) and R (R version 4.0.4).
Results
Baseline characteristics and outcome
Overall, 178 patients undergoing M-TEER for moderate-to-severe or severe SMR and sufficient echocardiographic MV assessment (long-axis cross-sectional views of all three MV segments) before the procedure were included. Patients mean age was 72 ± 11 years. The etiology of SMR was ischemic in 106 patients (59.2%). Mean LV-EF was impaired with 35.3 ± 11.2%. All patients had MR grade 3 + (59.6%) or 4 + (40.4%). LV-EDV and LV-ESV were 178.9 ± 69.9 ml and 117.0 ± 57.8 ml, respectively. Most patients presented highly symptomatic in NYHA functional class III (74.2%) or IV (25.7%). Average renal function was impaired in the majority of patients (estimated glomerular filtration rate [eGFR] 53.6 ± 22.8 ml/min). Clinical and echocardiographic baseline characteristics are shown in Table 1 and Table 2, respectively. Patients were treated using MitraClip NT (n = 148, 83.1%), NTR (n = 14, 7.9%), or XTR (n = 15, 8.4%). One patient (0.6%) received both, MitraClip NTR and XTR.
M-TEER led to a procedural decrease of MR severity to grade ≤ 1 + in 117 (65.7%) patients, and ≤ 2 + in 164 (92.1%) patients. Postprocedural MR was 3+ and 4+ in 12 patients (6.7%) and 2 patients (1.1%), respectively. Clinical follow-up rates in eligible patients were 88.8% at 1 year and 82.1% at 2 years. Overall survival rates were 80.4% and 70.6% at 1- and 2-year follow-up, respectively. NYHA class follow-up was available in 121 patients (70.0%).
Asymmetry of mitral valve leaflet tethering
Asymmetric postero-anterior MV tethering
Table 2 gives a detailed overview on the MV anatomy of the study cohort. Postero-anterior MV leaflet tethering asymmetry was defined as the PLA/ALA ratio in MV segment 2, as tethering was most pronounced within the central MV segment (Supplementary Table 1). Mean postero-anterior tethering asymmetry was 1.54 ± 1.22. The postero-anterior asymmetry was mainly driven by PML restriction (PLA: 48.2 ± 33.6°). ROC analysis revealed that postero-anterior S2 tethering {area under curve [AUC] = 0.61, CI 0.51–0.70, p = 0.03} provides discriminatory power regarding prediction of 2-year all-cause mortality. Subsequent calculation of Youden’s J identified a PLA/ALA ratio > 1.54 as best discriminatory value for asymmetric postero-anterior tethering regarding 2-year survival prognosis (sensitivity 0.53; specificity 0.68). Figure 2A graphically outlines the relationship of postero-anterior tethering symmetry and mortality risk after M-TEER. Within the range of PLA/ALA ratio 1.0:2.0, HR had a linear association, while PLA/ALA ratios < 1.0 and > 1.75 had a static association. Sixty-seven patients (37.6%) were identified to have asymmetric postero-anterior tethering with a PLA/ALA ratio > 1.54.
Cox regression spline curves for postero-anterior and medio-lateral tethering asymmetry. A Spline curve for postero-anterior tethering asymmetry. B Spline curve for medio-lateral tethering asymmetry. Postero-anterior and medio-lateral tethering asymmetry are represented by the PLA/ALA and S3/S1 tenting area ratios, respectively
Clinical baseline characteristics did not differ between patients with symmetric and asymmetric postero-anterior tethering, except for a higher prevalence of previous coronary artery bypass graft (CABG) surgery and coronary artery disease (CAD, both p = 0.042, Supplementary Table 1) in patients with asymmetric postero-anterior tethering. MR severity expressed by MR EROA, RegVol, and VC was comparable in both groups. A logistic regression model identified CAD {Odds ratio [OR] = 2.19, CI 1.13–4.23, p = 0.02} and MV mean PG (OR = 1.53, CI 1.13–4.23, p = 0.03) to be associated with asymmetric postero-anterior tethering (Supplementary Table 2). Implantation of at least one clip was successfully performed in 167 patients (93.8%) in the total cohort. Postprocedural MR remained higher in patients with asymmetric postero-anterior tethering (p < 0.01), while there was no difference at baseline (Fig. 3, Supplementary Table 3). Among a total of 14 patients with residual MR ≥ 3 + , 10 did not receive any device. The main reason was development of high MV pressure gradients after device positioning (mean gradient before clip retraction: 8.3 ± 1.8 mmHg). In two patients, no device was implanted due to a short, immobile posterior leaflets with extensive asymmetric postero-anterior tethering. Four patients received at least one device. One patient suffered from device detachment of the second implanted clip with the residual MR jet being located near the device. In three patients, residual MR remained ≥ 3 + , after positioning of the first clip. Due to development of high MV pressure gradients after positioning of another clip in the presence of asymmetric tethering, MR could not further be reduced. In these cases, residual MR was located near the already implanted device. Sensitivity analysis identified a PLA/ALA ratio > 1.80 as optimal cut-off for the association with residual postprocedural MR (AUC = 0.69, CI 0.52–0.86, p = 0.03).
MR reduction in patients with symmetric and asymmetric postero-anterior leaflet tethering. Asymmetric postero-anterior MV tethering is associated with less profound procedural MR reduction in patients undergoing M-TEER for severe MR. MV mitral valve; MR mitral regurgitation; M-TEER transcatheter mitral valve edge-to-edge repair. 178 paired samples
Asymmetric medio-lateral MV tethering
Medio-lateral MV leaflet tethering asymmetry was defined as the ratio of the MV tenting area in MV segment 3 compared to segment 1. ROC analysis and subsequent calculation of Youden’s J revealed an S3/S1 ratio > 1.49 as being associated with survival; thus, we termed this pattern asymmetric medio-lateral tethering (AUC = 0.66, CI 0.56–0.75, p < 0.01; sensitivity 0.48, specificity 0.81). Figure 2B depicts the rather cubic than linear relationship of medio-lateral tethering asymmetry and mortality risk after M-TEER. Forty-nine (27.5%) patients presented with asymmetric medio-lateral tethering.
Of note, there were no between-group differences in clinical or echocardiographic parameters at baseline (e.g., MR EROA, RegVol, VC), except for a higher degree of TR severity in patients with asymmetric medio-lateral tethering (Supplementary Tables 1 and 3). Correspondingly, sensitivity analysis did not identify a significant cut-off for the association of medio-lateral tethering symmetry and residual MR (AUC = 0.43, CI 0.26–0.60, p = 0.39). Furthermore, logistic regression did not identify parameters being independently associated with asymmetric medio-lateral tethering (Supplementary Table 4).
Prognostic value of asymmetric postero-anterior and medio-lateral MV tethering
Predictors for all-cause 2-year mortality in the univariable analysis are summarized in Supplementary Table 5. Multivariable Cox regression revealed LV-EF (per 10% decrease, HR = 1.42, 95% CI 1.04–1.94 p = 0.03), eGFR (per 10 ml/min decrease, HR = 1.25, 95% CI 1.06–1.47, p = 0.01), prior CABG (HR = 2.30, 95% CI 1.13–4.68, p = 0.02), extracardiac arteriopathy (HR = 2.63, 95% CI 1.19–5.78, p = 0.02), asymmetric postero-anterior leaflet tethering (HR = 2.77, CI 1.43–5.38, p = 0.01), and asymmetric medio-lateral tethering (HR = 2.90, CI 1.54–5.45, p < 0.01) to be associated with increased all-cause 2-year mortality (Table 3 and Fig. 4). Patients with concomitant asymmetric postero-anterior and medio-lateral tethering showed particularly reduced 1- and 2-year survival rates compared to those with either asymmetric postero-anterior or medio-lateral tethering or patients without any asymmetric tethering pattern (Graphic abstract).
Multivariable Cox model for 2-year all-cause mortality. Predictors for 2-year all-cause mortality after M-TEER for SMR. eGFR per 10 ml/min decrease, LV-EF per 10% decrease; eGFR estimated glomerular filtration rate; LV-EF left-ventricular ejection fraction; LV-ESV left-ventricular end-systolic volume; HR hazard ratio; NYHA New York Heart Association; TAPSE tricuspid annular plane systolic excursion; M-TEER transcatheter mitral valve edge-to-edge repair; SMR secondary mitral regurgitation, n = 164
NYHA class at baseline did not differ in patients with symmetric vs asymmetric postero-anterior and medio-lateral tethering. We observed NYHA class improvement independent of any leaflet tethering pattern (both p < 0.001; Fig. 5A, B).
NYHA functional class development. NYHA functional class at baseline and at follow-up examination in patients with symmetric vs asymmetric postero-anterior (A) and medio-lateral MV leaflet tethering (B). NYHA New York Heart Association; MV mitral valve. Postero-anterior tethering: 83 paired samples. Medio-lateral tethering: 38 paired samples
Discussion
With more than 10 years of experience, M-TEER is the most widely applied transcatheter treatment approach for high-risk patients with severe SMR. Safety, efficacy, and symptomatic long-term benefit have been shown in several studies [3,4,5, 24,25,26]. In this study, we report about the impact of asymmetric MV leaflet tethering on procedural, symptomatic, and prognostic outcomes in patients undergoing M-TEER for severe SMR.
Asymmetric postero-anterior leaflet tethering was present in about 38% of patients. So far, data on the impact of asymmetric tethering patterns on outcomes after M-TEER are sparse. One study identified restricted PML leaflet tethering to be tightly associated with M-TEER failure defined as MR ≥ 3 + 12 months after intervention [18]. Extending these results, we identified asymmetric postero-anterior tethering to be predominantly caused by excessive PML restriction in comparison to moderately impaired AML systolic motion. Consistently, we found postprocedural MR severity to be significantly higher in the presence of asymmetric postero-anterior tethering, despite a comparable baseline MR. Hence, a more eccentric preprocedural MR jet direction may contribute to higher postprocedural MR rates [27].
Asymmetric medio-lateral tethering was associated with substantially elevated mortality rates in this study. In the literature, asymmetric medio-lateral tethering is considered to be a consequence of inferoposterior myocardial infarction and subsequent apical papillary muscle displacement [15].
This is the first study to assess the impact of asymmetric MV leaflet tethering on mortality after M-TEER for SMR. We identified both asymmetric postero-anterior and medio-lateral tethering to be associated with increased all-cause 2-year mortality besides known clinical predictors as left-ventricular and renal function [5]. Patients with a severely distorted valve anatomy comprising concomitant asymmetric postero-anterior and medio-lateral tethering had the worst 2-year survival. Importantly, symptomatic improvement assessed by NYHA functional did not differ between groups of tethering patterns.
We hypothesise that asymmetric postero-anterior MV tethering leads to worse procedural MR reduction and therefore is associated with inferior survival outcome after M-TEER. For asymmetric medio-lateral tethering, the exact pathophysiological link is less obvious. Patients with the latter condition might represent a certain etiological subgroup of SMR patients, who experience unfavourable ventricular remodelling within their underlying cardiomyopathic disease process. Apical displacement of the medial papillary muscle could contribute to this observation, as this unilateral affection of a major subvalvular structure could lead to asymmetric distortion of the MV as expressed by medio-lateral tethering. Nevertheless, further studies (e.g., animal models) are needed to clarify the exact pathophysiologic mechanism.
The current study outlines the importance of asymmetric MV leaflet tethering on both procedural MR reduction and mortality following a transcatheter treatment approach for severe SMR. In MV surgery, both asymmetric postero-anterior and medio-lateral tethering have been identified as conditions associated with higher rates of residual MR, and mortality [19, 20, 28]. Within the past years, novel surgical techniques were developed to tackle this challenge. Subannular repair techniques provide the possibility to reduce the degree of postprocedural tethering and follow-up MR compared to the conventional isolated annuloplasty [29]. Current application of surgical techniques include subannular strategies in addition to undersizing annuloplasty, which suggests a benefit for left-ventricular remodelling and MR recurrence compared to annuloplasty alone [30,31,32], although this strategy has to be evaluated in randomized trials. All patients included into this analysis were treated using devices without the possibility of independent leaflet grasping. This led to difficulties in grasping the MV leaflets, especially in patients with asymmetric postero-anterior tethering without generating significant MV stenosis. Advanced generations of M-TEER devices (e.g., the PASCAL system [Edwards Lifesciences, Irvine, California] and the fourth-generation MitraClip devices) provide the possibility of independent leaflet grasping [33]. This novel feature might facilitate appropriate leaflet grasping and effective MR reduction even in patients with complicated tethering patterns without generation of significant MV stenosis. Beyond that, the development of transapical or transcatheter MV replacement devices may provide an important treatment alternative for patients with complex MV anatomy. Further studies are needed to optimize the therapeutic pathway for suffering from SMR due to asymmetric MV tethering. A head-to-head comparison of M-TEER vs. surgical MV repair/replacement in HFrEF patients is needed to evaluate the non-inferiority or even superiority between these approaches. This question is currently investigated in the ongoing randomized Mitral Valve Reconstruction For Advanced Insufficiency Of Functional Or Ischemic Origin (MATTERHORN) trial.
Limitations
This is a retrospective, single-center analysis of M-TEER treated patients without core-lab supervision whose follow-up data were acquired prospectively. Three-dimensional and multiple high-quality biplane two-dimensional imaging in various planes are state-of-the-art echocardiography in evaluating MV anatomy and function, which was necessary to perform the current analyses. Accordingly, a few patients, in whom such detailed imaging was not available, had to be excluded from this analysis. Even after application of a multivariable Cox model, we cannot rule out that further parameters not included into the manuscript may have an impact on the predictive value of asymmetric tethering patterns. Due to the retrospective character of this study, no information on the exact localization of previous MI was available. Since patients with asymmetric tethering had worse survival after M-TEER, there could be bias for NYHA follow-up examination. We further acknowledge that the newly defined cut-offs for the definition of postero-anterior and medio-lateral tethering need further prospective validation in larger M-TEER patient cohorts, especially in the light of possible differences in baseline characteristics between patients.
Conclusions
Asymmetric tethering of the mitral valve leaflets—either in the postero-anterior or medio-lateral direction—is identified as strong independent predictors for all-cause 2-year mortality in patients with severe SMR undergoing M-TEER. Accordingly, we recommend including the precise evaluation of asymmetric tethering patterns into the routine pre-interventional assessment of SMR to account for potential challenges in MR reduction as well as considering alternative devices including transcatheter mitral valve implantation which may be associated with improved outcomes in such patients. Further randomized-controlled trials are needed to compare different interventional MV repair devices and techniques.
Data availability
All authors had unrestricted access to the complete data.
References
Asgar AW, Mack MJ, Stone GW (2015) Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 65:1231–1248. https://doi.org/10.1016/j.jacc.2015.02.009
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C (2020) 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. https://doi.org/10.1016/j.jacc.2020.11.018
Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ (2018) Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 379:2307–2318. https://doi.org/10.1056/NEJMoa1806640
Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefevre T, Piot C, Rouleau F, Carrie D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort-Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N (2018) Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 379:2297–2306. https://doi.org/10.1056/NEJMoa1805374
Orban M, Orban M, Lesevic H, Braun D, Deseive S, Sonne C, Hutterer L, Grebmer C, Khandoga A, Pache J, Mehilli J, Schunkert H, Kastrati A, Hagl C, Bauer A, Massberg S, Boekstegers P, Nabauer M, Ott I, Hausleiter J (2017) Predictors for long-term survival after transcatheter edge-to-edge mitral valve repair. J Interv Cardiol 30:226–233. https://doi.org/10.1111/joic.12376
Karam N, Stolz L, Orban M, Deseive S, Praz F, Kalbacher D, Westermann D, Braun D, Näbauer M, Neuss M, Butter C, Kassar M, Petrescu A, Pfister R, Iliadis C, Unterhuber M, Park SD, Thiele H, Baldus S, Stephan von Bardeleben R, Blankenberg S, Massberg S, Windecker S, Lurz P, Hausleiter J (2021) Impact of right ventricular dysfunction on outcomes after transcatheter edge-to-edge repair for secondary mitral regurgitation. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2020.12.015
Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ (2001) Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 103:1759–1764. https://doi.org/10.1161/01.cir.103.13.1759
Bartko PE, Arfsten H, Heitzinger G, Pavo N, Toma A, Strunk G, Hengstenberg C, Hülsmann M, Goliasch G (2019) A unifying concept for the quantitative assessment of secondary mitral regurgitation. J Am Coll Cardiol 73:2506–2517. https://doi.org/10.1016/j.jacc.2019.02.075
Gelsomino S, van Garsse L, Lucà F, Parise O, Cheriex E, Rao CM, Gensini GF, Maessen J (2013) Left ventricular strain in chronic ischemic mitral regurgitation in relation to mitral tethering pattern. J Am Soc Echocardiogr 26:370-380.e11. https://doi.org/10.1016/j.echo.2013.01.011
He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA (1997) Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation 96:1826–1834. https://doi.org/10.1161/01.cir.96.6.1826
El Sabbagh A, Reddy YNV, Nishimura RA (2018) Mitral valve regurgitation in the contemporary era: insights into diagnosis, management, and future directions. JACC Cardiovasc Imaging 11:628–643. https://doi.org/10.1016/j.jcmg.2018.01.009
Deferm S, Bertrand PB, Verbrugge FH, Verhaert D, Rega F, Thomas JD, Vandervoort PM (2019) Atrial functional mitral regurgitation: JACC review topic of the week. J Am Coll Cardiol 73:2465–2476. https://doi.org/10.1016/j.jacc.2019.02.061
Tamargo M, Obokata M, Reddy YNV, Pislaru SV, Lin G, Egbe AC, Nishimura RA, Borlaug BA (2020) Functional mitral regurgitation and left atrial myopathy in heart failure with preserved ejection fraction. Eur J Heart Fail 22:489–498. https://doi.org/10.1002/ejhf.1699
Stolz L, Orban M, Braun D, Stark K, Steffen J, Orban M, Hagl C, Massberg S, Näbauer M, Hausleiter J (2021) Anatomy and outcome of secondary mitral regurgitation subtypes undergoing transcatheter mitral valve edge-to-edge repair. JACC Cardiovasc Interv 14:110–111. https://doi.org/10.1016/j.jcin.2020.09.035
Kim K, Kaji S, An Y, Yoshitani H, Takeuchi M, Levine RA, Otsuji Y, Furukawa Y (2012) Mechanism of asymmetric leaflet tethering in ischemic mitral regurgitation: 3D analysis with multislice CT. JACC Cardiovasc Imaging 5:230–232. https://doi.org/10.1016/j.jcmg.2011.08.023
Zeng X, Nunes MC, Dent J, Gillam L, Mathew JP, Gammie JS, Ascheim DD, Moquete E, Hung J (2014) Asymmetric versus symmetric tethering patterns in ischemic mitral regurgitation: geometric differences from three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr 27:367–375. https://doi.org/10.1016/j.echo.2014.01.006
Agricola E, Oppizzi M, Maisano F, De Bonis M, Schinkel AF, Torracca L, Margonato A, Melisurgo G, Alfieri O (2004) Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr 5:326–334. https://doi.org/10.1016/j.euje.2004.03.001
Taramasso M, Denti P, Latib A, Guidotti A, Buzzatti N, Pozzoli A, Di Giannuario G, La Canna G, Colombo A, Alfieri O, Maisano F (2015) Clinical and anatomical predictors of MitraClip therapy failure for functional mitral regurgitation: single central clip strategy in asymmetric tethering. Int J Cardiol 186:286–288. https://doi.org/10.1016/j.ijcard.2015.03.236
Kron IL, Hung J, Overbey JR, Bouchard D, Gelijns AC, Moskowitz AJ, Voisine P, O’Gara PT, Argenziano M, Michler RE, Gillinov M, Puskas JD, Gammie JS, Mack MJ, Smith PK, Sai-Sudhakar C, Gardner TJ, Ailawadi G, Zeng X, O’Sullivan K, Parides MK, Swayze R, Thourani V, Rose EA, Perrault LP, Acker MA (2015) Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 149:752–61.e1. https://doi.org/10.1016/j.jtcvs.2014.10.120
Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, Hung JW, Voisine P, Dagenais F, Gillinov AM, Thourani V, Argenziano M, Gammie JS, Mack M, Demers P, Atluri P, Rose EA, O’Sullivan K, Williams DL, Bagiella E, Michler RE, Weisel RD, Miller MA, Geller NL, Taddei-Peters WC, Smith PK, Moquete E, Overbey JR, Kron IL, O’Gara PT, Acker MA (2016) Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med 374:344–353. https://doi.org/10.1056/NEJMoa1512913
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL (2017) 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 38:2739–2791. https://doi.org/10.1093/eurheartj/ehx391
Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL, Scientific Document Committee of the European Association of Cardiovascular I (2013) Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 14:611–644. https://doi.org/10.1093/ehjci/jet105
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL (2017) 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 38:2739–2791. https://doi.org/10.1093/eurheartj/ehx391
Attizzani GF, Ohno Y, Capodanno D, Cannata S, Dipasqua F, Imme S, Mangiafico S, Barbanti M, Ministeri M, Cageggi A, Pistritto AM, Giaquinta S, Farruggio S, Chiaranda M, Ronsivalle G, Schnell A, Scandura S, Tamburino C, Capranzano P, Grasso C (2015) Extended use of percutaneous edge-to-edge mitral valve repair beyond EVEREST (endovascular valve edge-to-edge repair) criteria: 30-day and 12-month clinical and echocardiographic outcomes from the GRASP (getting reduction of mitral insufficiency by percutaneous clip implantation) registry. JACC Cardiovasc Interv 8:74–82. https://doi.org/10.1016/j.jcin.2014.07.024
Glower DD, Kar S, Trento A, Lim DS, Bajwa T, Quesada R, Whitlow PL, Rinaldi MJ, Grayburn P, Mack MJ, Mauri L, McCarthy PM, Feldman T (2014) Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol 64:172–181. https://doi.org/10.1016/j.jacc.2013.12.062
Rudolph V, Knap M, Franzen O, Schluter M, de Vries T, Conradi L, Schirmer J, Treede H, Wegscheider K, Costard-Jackle A, Meinertz T, Reichenspurner H, Baldus S (2011) Echocardiographic and clinical outcomes of MitraClip therapy in patients not amenable to surgery. J Am Coll Cardiol 58:2190–2195. https://doi.org/10.1016/j.jacc.2011.07.047
Utsunomiya H, Itabashi Y, Kobayashi S, Yoshida J, Ikenaga H, Rader F, Hussaini A, Makar M, Trento A, Siegel RJ, Kar S, Shiota T (2019) Comparison of mitral valve geometrical effect of percutaneous edge-to-edge repair between central and eccentric functional mitral regurgitation: clinical implications. Eur Heart J Cardiovasc Imaging 20:455–466. https://doi.org/10.1093/ehjci/jey117
Daimon M, Fukuda S, Adams DH, McCarthy PM, Gillinov AM, Carpentier A, Filsoufi F, Abascal VM, Rigolin VH, Salzberg S, Huskin A, Langenfeld M, Shiota T (2006) Mitral valve repair with Carpentier-McCarthy-Adams IMR ETlogix annuloplasty ring for ischemic mitral regurgitation: early echocardiographic results from a multi-center study. Circulation 114:I588–I593. https://doi.org/10.1161/circulationaha.105.001347
Harmel E, Pausch J, Gross T, Petersen J, Sinning C, Kubitz J, Reichenspurner H, Girdauskas E (2019) Standardized subannular repair improves outcomes in type IIIb functional mitral regurgitation. Ann Thorac Surg 108:1783–1792. https://doi.org/10.1016/j.athoracsur.2019.04.120
Mihos CG, Xydas S, Yucel E, Capoulade R, Williams RF, Mawad M, Garcia G, Santana O (2017) Mitral valve repair and subvalvular intervention for secondary mitral regurgitation: a systematic review and meta-analysis of randomized controlled and propensity matched studies. J Thorac Dis 9:S582–S594. https://doi.org/10.21037/jtd.2017.05.56
Calafiore AM, Refaie R, Iacò AL, Asif M, Al Shurafa HS, Al-Amri H, Romeo A, Di Mauro M (2014) Chordal cutting in ischemic mitral regurgitation: a propensity-matched study. J Thorac Cardiovasc Surg 148:41–46. https://doi.org/10.1016/j.jtcvs.2013.07.036
Yamaguchi A, Adachi K, Yuri K, Kimura N, Kimura C, Tamura A, Adachi H (2013) Reduction of mitral valve leaflet tethering by procedures targeting the subvalvular apparatus in addition to mitral annuloplasty. Circ J 77:1461–1465. https://doi.org/10.1253/circj.cj-12-1148
Chakravarty T, Makar M, Patel D, Oakley L, Yoon SH, Stegic J, Singh S, Skaf S, Nakamura M, Makkar RR (2020) Transcatheter edge-to-edge mitral valve repair with the MitraClip G4 system. JACC Cardiovasc Interv 13:2402–2414. https://doi.org/10.1016/j.jcin.2020.06.053
Acknowledgements
We thank Diana Rösler, Andrea Englmaier, Stephanie Menner, and Tobias Reithmayer for their extensive support over the course of the study.
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mathias Orban received speaker fees from Abbott Vascular and Tomtec Imaging Systems. Michael Näbauer and Daniel Braun have received speaker honoraria from Abbott Vascular. Martin Orban received Speaker honoraria from Abbott Medical, AstraZeneca, Abiomed, Bayer vital, Biotronik, Bristol-Myers Squibb, CytoSorbents, Daiichi Sankyo Deutschland, Edwards Lifesciences Services, and Sedana Medical. Jörg Hausleiter has received speaker honoraria as well as research support from and serves as consultant for Abbott Vascular and for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Ethical approval
This retrospective study was approved by the local ethics committee and conforms with the 1964 Declaration of Helsinki and its later amendments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stolz, L., Orban, M., Braun, D. et al. Impact of asymmetric tethering on outcomes after edge-to-edge mitral valve repair for secondary mitral regurgitation. Clin Res Cardiol 111, 869–880 (2022). https://doi.org/10.1007/s00392-021-01961-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-021-01961-5