Abstract
Aims
We sought to assess the impact of different manifestations of heart failure (HF) at baseline on the short- and long-term outcomes of transcatheter aortic valve implantation (TAVI) for aortic stenosis (AS).
Methods and results
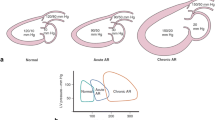
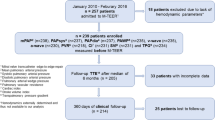
Of 361 patients undergoing TAVI between May 2013 and April 2015, 185 (51%) showed clinical signs of HF at the time of admission. HF was diagnosed as isolated left ventricular (LV) and biventricular in 63 (34%) and 122 patients (66%), respectively. Acute device success (VARC-2) was achieved in 97% of patients without HF, in all patients with LV HF, and in 97% of patients with biventricular HF. Follow-up for a median of 427 days revealed significantly poorer survival in patients with biventricular HF (1-year estimate, 72.1% [95% confidence interval, 64.0–80.2%]) than in patients with LV HF (84.5% [75.2–93.8%]; p = 0.0203) or no HF (94.3% [90.7–97.9%]; p < 0.0001). Survival in the latter two patient subgroups was statistically not different. A diagnosis of biventricular HF was associated with a hazard ratio of 2.62 (p = 0.0089) vs. no HF in the likelihood of death; NT-proBNP and the logistic EuroSCORE were not significantly associated with survival. Half of all deaths in patients with biventricular HF occurred within 42 days of TAVI.
Conclusion
Biventricular HF is a strong predictor of mortality following TAVI for severe AS. AS in patients with LV HF should be treated without delay to avoid progression to biventricular HF. Patients with AS and biventricular HF should be monitored closely after TAVI to possibly prevent early death.

Similar content being viewed by others
References
Ponikowski P, Voors AA, Anker SD et al (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 37:2129–2200
Rosenhek R, Zilberszac R, Schemper M et al (2010) Natural history of very severe aortic stenosis. Circulation 121:151–156
Rosenhek R, Binder T, Porenta G et al (2000) Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 343:611–617
Pellikka PA, Sarano ME, Nishimura RA et al (2005) Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 111:3290–3295
Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA (2005) Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 112:I377–I382
Bergler-Klein J, Klaar U, Heger M et al (2004) Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 109:2302–2308
Marechaux S, Hachicha Z, Bellouin A et al (2010) Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 31:1390–1397
Baumgartner H, Falk V, Bax JJ et al (2017) 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 10:893–853
Frank S, Johnson A, Ross J (1973) Natural history of valvular aortic stenosis. Br Heart J 35:41–46
Thiele H, Zeymer U, Neumann F-J et al (2012) Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 367:1287–1296
Smith CR, Leon MB, Mack MJ et al (2011) Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364:2187–2198
Grube E, Laborde JC, Gerckens U et al (2006) Percutaneous implantation of the corevalve self-expanding valve prosthesis in high-risk patients with aortic valve disease. Circulation 114(15):1616–1624
Kappetein AP, Head SJ, Genereux P et al (2012) Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 33:2403–2418
R Core Team (2018) R:A language and environment for statistical computing. Available at: https://www.R-project.org. Accessed 20 Mar 2018
Malaisrie SC, McDonald E, Kruse J et al (2014) Mortality while waiting for aortic valve replacement. Ann Thorac Surg 98:1564–1570 :– discussion 1570–1.
Landes U, Orvin K, Codner P et al (2016) Urgent transcatheter aortic valve implantation in patients with severe aortic stenosis and acute heart failure: procedural and 30-day outcomes. Can J Cardiol 32:726–731
Frerker C, Schewel J, Schluter M et al (2016) Emergency transcatheter aortic valve replacement in patients with cardiogenic shock due to acutely decompensated aortic stenosis. EuroIntervention 11:1530–1536
Van Ancum JM, Scheerman K, Pierik VD et al (2017) Muscle strength and muscle mass in older patients during hospitalization: the EMPOWER Study. Gerontology 63:507–514
Boumendil A, Somme D, Garrouste-Orgeas M, Guidet B (2007) Should elderly patients be admitted to the intensive care unit? Intensive Care Med 33:1252
Vidán MT, Sánchez E, Fernández-Avilés F, Serra-Rexach JA, Ortiz J, Bueno H (2014) FRAIL-HF, a study to evaluate the clinical complexity of heart failure in nondependent older patients: rationale, methods and baseline characteristics. Clin Cardiol 37:725–732
Suissa S, Dell’Aniello S, Ernst P (2012) Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 67:957–963
Damman K, Valente MAE, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL (2014) Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 35:455–469
Filippatos G, Farmakis D, Parissis J (2014) Renal dysfunction and heart failure: things are seldom what they seem. Eur Heart J 35:416–418
Schewel J, Schewel D, Frerker C, Wohlmuth P, Kuck K-H, Schäfer U (2016) Invasive hemodynamic assessments during transcatheter aortic valve implantation: comparison of patient outcomes in higher vs. lower transvalvular gradients with respect to left ventricular ejection fraction. Clin Res Cardiol 105:59–71
Schymik G, Tzamalis P, Herzberger V et al (2017) Transcatheter aortic valve implantation in patients with a reduced left ventricular ejection fraction: a single-centre experience in 2000 patients (TAVIK Registry). Clin Res Cardiol 106:1018–1025
Fraccaro C, Al-Lamee R, Tarantini G et al. (2012) Transcatheter aortic valve implantation in patients with severe left ventricular dysfunction: immediate and mid-term results, a multicenter study. Circ Cardiovasc Interv 5:253–260
Gotzmann M, Rahlmann P, Hehnen T et al (2012) Heart failure in severe aortic valve stenosis: prognostic impact of left ventricular ejection fraction and mean gradient on outcome after transcatheter aortic valve implantation. Eur J Heart Fail 14:1155–1162
Eichler S, Salzwedel A, Harnath A et al (2018) Nutrition and mobility predict all-cause mortality in patients 12 months after transcatheter aortic valve implantation. Clin Res Cardiol 107:304–311
Arsalan M, Filardo G, Kim W-K et al (2016) Prognostic value of body mass index and body surface area on clinical outcomes after transcatheter aortic valve implantation. Clin Res Cardiol 105:1042–1048
Abdelghani M, Cavalcante R, Miyazaki Y et al (2017) Prevalence, predictors, and prognostic implications of residual impairment of functional capacity after transcatheter aortic valve implantation. Clin Res Cardiol 106:752–759
Funding
This work did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tobias Schmidt has received lecture honoraria from Medtronic, as well as travel expenses from Edwards LifeSciences, Medtronic, and Boston Scientific. Christian Frerker has received lecture honoraria and travel expenses from Medtronic, Edwards Lifesciences, and Abbott Vascular. Karl-Heinz Kuck has received consultation fees from Medtronic, Boston Scientific, Biosense Webster, Edwards LifeSciences, and Abbott Vascular. The other authors report no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmidt, T., Bohné, M., Schlüter, M. et al. The impact of biventricular heart failure on outcomes after transcatheter aortic valve implantation. Clin Res Cardiol 108, 741–748 (2019). https://doi.org/10.1007/s00392-018-1400-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1400-6