Abstract
Background
Heart failure is heterogeneous in aetiology, pathophysiology, and presentation. Despite this diversity, clinical trials of patients hospitalized for HF deal with this problem as a single entity, which may be one reason for repeated failures.
Methods
The first EuroHeart Failure Survey screened consecutive deaths and discharges of patients with suspected heart failure during 2000–2001. Patients were sorted into seven mutually exclusive hierarchical presentations: (1) with cardiac arrest/ventricular arrhythmia; (2) with acute coronary syndrome; (3) with rapid atrial fibrillation; (4) with acute breathlessness; (5) with other symptoms/signs such as peripheral oedema; (6) with stable symptoms; and (7) others in whom the contribution of HF to admission was not clear.
Results
The 10,701 patients enrolled were classified into the above seven presentations as follows: 260 (2%), 560 (5%), 799 (8%), 2479 (24%), 1040 (10%), 703 (7%), and 4691 (45%) for which index-admission mortality was 26%, 20%, 10%, 8%, 6%, 6%, and 4%, respectively. Compared to those in group 7, the hazard ratios for death during the index admission were 4.9 (p ≤ 0.001), 4.0 (p < 0.001), 2.2 (p < 0.001), 2.1 (p < 0.001), 1.4 (p < 0.04) and 1.4 (p = 0.04), respectively. These differences were no longer statistically significant by 12 weeks.
Conclusion
There is great diversity in the presentation of heart failure that is associated with very different short-term outcomes. Only a minority of hospitalizations associated with suspected heart failure are associated with acute breathlessness. This should be taken into account in the design of future clinical trials.
Similar content being viewed by others
Introduction
Acute heart failure (AHF) is heterogeneous in its aetiology, pathophysiology, and presentation. European Society of Cardiology (ESC) 2008 guidelines classify AHF into six different clinical presentations, amongst which there is considerable overlap. Most events are classified as decompensated chronic heart failure and the rest as acute pulmonary oedema, cardiogenic shock, right heart failure or associated with severe hypertension or acute coronary syndrome [1]. It is clear that AHF is not a discrete diagnosis but a collection of different clinical syndromes that require clinical intervention with varying degrees of urgency [2, 3]. A patient with severe breathlessness at rest in acute pulmonary oedema is a medical emergency requiring immediate investigation and treatment [4, 5]. A patient presenting with increasing exertional breathlessness and worsening peripheral oedema might be considered sub-acute and requiring treatment within hours or days rather than minutes [6, 7]. If the target and purpose of therapy is diverse then trials that treat all AHF as a single entity are likely to fail. Better characterization of the heterogeneous clinical presentation of AHF might help inform the design of future clinical trials that target the unmet needs of specific presentations of AHF [8,9,10]. Accordingly, we obtained information from the First EuroHeart Failure Survey (EHFS-1) that enrolled more than 10,000 patients from 115 hospitals over a 6 week snapshot to describe the outcome of patients with different presentations of patients hospitalized for or with heart failure [11, 12].
Methods
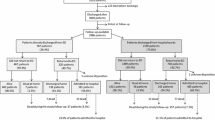
EHFS-1 screened consecutive deaths and discharges during 2000–2001 primarily from medical wards over a 6 week period in 115 hospitals from 24 countries in Europe, to identify patients with known or suspected heat failure (HF). The design and implementation of the survey have been published in detail previously [13]. Each hospital recorded consecutive deaths and discharges from medical, cardiology, cardiac surgery, and geriatric medicine wards over a period of 6 weeks [11]. Surgical, gynaecology, ophthalmology, and renal wards were excluded. Records were screened to identify if the patient fulfilled one or more of the following four criteria:
-
1.
A clinical diagnosis of HF, primary or contributory, during the index admission.
-
2.
A diagnosis of HF recorded in the hospital records at any time during the previous 3 years.
-
3.
Administration of loop diuretics during the 24 h prior to death or discharge during the index admission, other than for end-stage renal disease.
-
4.
Administration of treatment for HF or for ventricular dysfunction within the 24 h prior to death or discharge.
Patients who fulfilled one or more inclusion criteria were further classified by investigators according to clinical presentation, aetiology, final diagnosis and whether, in the investigators opinion, HF was the primary diagnosis, a secondary diagnosis complicating or prolonging hospital admission, an incidental finding (e.g. patients who were admitted for another reason and whose admission was not complicated or prolonged by HF) or diagnostically uncertain (mostly patients taking loop diuretics for no obvious reason).
Classification of presentation
Presentation at hospital admission was classified hierarchically (patients belonging to a preceding class/group could not belong to any subsequent class/group) as follows:
-
1.
Cardiac arrest, ventricular tachycardia, or fibrillation or cardiogenic shock.
-
2.
Acute coronary syndrome (ACS).
-
3.
Atrial fibrillation (AF) with a rapid ventricular response (> 120/min).
-
4.
Severe shortness of breath at rest.
-
5.
Other symptoms of HF, such as worsening peripheral oedema.
-
6.
Stable symptoms.
-
7.
Contribution of HF to admission uncertain.
Detailed information regarding events contributing to the current admission, cardiovascular and non-cardiovascular comorbid illnesses, and clinical investigations during admission and therapy at discharge or 24 h prior to death were recorded by investigators as well as deaths during the index hospital admission and deaths and readmissions within 12 weeks after discharge.
For most patients, left ventricular systolic dysfunction (LVSD) was not measured formally but assessed in a semi-quantitative fashion. For guidance, a left ventricular ejection fraction (LVEF) of < 40% was considered to reflect moderate-to-severe LVSD.
Continuous data are summarized by the median and 25th/75th percentiles; categorical data by percentages. As time-to-event data were not recorded after discharge, prognostic models for all-cause mortality were developed using Logistic regression. Prognostic models were developed using k-fold cross validation [14]. This procedure splits the data randomly into k partitions. For each partition, it fits the specified model using the other k − 1 groups, and uses the resulting parameters to predict the dependent variable in the unused group [15, 16]. We arbitrarily choose k as 25 (hence 25-fold cross validation). We started with 50 clinically relevant variables and then selected those variables in the final model that remained significant for at least 70% of cross validations. The significance level to remain in the model was initially set to 0.05 for each model. From the logistic regression models, receiver operating characteristic (ROC) curves were plotted as sensitivity versus 1 − specificity. An area under the ROC curve was calculated using methods outlined in Hanley and McMeil [17]. The area under the ROC represents the probability of classifying an individual as dead/alive. An area under the ROC curve of 1.0 means perfect classification, while an area of 0.5 means that classification is no better than chance. The Stata 13 statistical computer package was used to analyse the data.
Results
HF was the primary diagnosis in 4234 (40%) patients, a secondary diagnosis in 1772 (17%), and was considered not to have caused or complicated the index admission in 4695 (44%) [18]. Of the 6006 patients in whom HF was thought to cause or complicate admission, the most common presentation was severe breathlessness at rest (n = 2479; 42% of such patients) (Table 1).
The age and sex distribution was broadly similar for the various patient presentations, but patients presenting with a cardiac arrest or in shock and those with stable symptoms were slightly younger and were more likely to be men (Table 1). Apart from those admitted with a cardiac arrest/shock or ACS, about one-third of patients had a prior history of myocardial infarction. AF was present in 34–43% of patients, not including those patients with rapid AF as their primary presentation. For each group, apart from ACS, > 50% had been treated with loop diuretics prior to admission and prescription at discharge ranged from 72 to 88% amongst groups. When echocardiographic information was available (6096 patients), moderate-to-severe left ventricular systolic dysfunction (LVSD) was reported in 41–69% of cases and moderate-to-severe mitral regurgitation in 25–42% (Table 1). Even when the contribution of HF to admission was uncertain, 41% were reported to have moderate-to-severe LVSD, 25% moderate-to-severe mitral regurgitation, 61% cardiomegaly or pulmonary congestion on a chest X-ray, 74% were prescribed loop diuretics, 60% an ACE inhibitor or angiotensin receptor blocker, and 38% a beta-blocker (Table 2), making a diagnosis of heart failure likely in many cases.
Mortality during the index admission was higher for those presenting with a ventricular arrhythmia, cardiac arrest or shock (26%), or an ACS (20%) (Table 2). The group for which HF made an uncertain contribution to admission had a lower in-patient mortality (4%). Median length of stay varied from 8 to 11 days amongst groups. Of 10,701 patients, 12 weeks follow-up data were available for 9779 (91%). Mortality in the 12 weeks after discharge varied little amongst groups, ranging from 5 to 8% (Table 2). Most of these deaths were ascribed to cardiovascular causes or infection; few were ascribed to cancer. Within 12 weeks, 19–29% of patients had been re-admitted, mostly for cardiovascular problems of which HF was the main reason in about half of admissions except for the group with an uncertain contribution of HF to the index admission.
On multivariable analysis, MI during the index admission, a history of VT/VF, a history of stroke, left ventricular dilatation, and serum creatinine concentration were associated with mortality on the index admission (Table 3). The area under receiver operating characteristics (ROC) curve was 0.74. A second model, using a less rigorous p value of 0.1 for selection, identified four more variables; age, sex, medical history of hypertension and infection. The area under ROC curve in the second model was 0.78 (Fig. 1). We used Akaike’s information criterion (AIC) and Bayesian information criterion (BIC) to compare the two models. A smaller AIC/BIC ratio in model 2 (0.96 in model 1 and 0.95 in model 2) and a large difference of BIC between models (67) indicated the superiority of model 2 [19, 20]. In a final logistic regression model after adjusting for all relevant covariates, mortality remained higher in groups 1–6 as compared to group 7 (uncertain contribution of HF to admission).
For mortality between discharge and 12 weeks, a history of valve repair was the only variable which was significant using p < 0.05 for model selection in 25 cross validations and the model discrimination was poor (ROC 0.55). If p < 0.1 was used, a history of diabetes, LVSD, left ventricle dilatation, and history of angina during index admission provided additional information but this improved the ROC only to 0.57. In the logistic regression model, only Group 2 (ACS) (OR 1.73, p = 0.03, CI 1.07–2.95) and Group 4 (severe breathlessness) (OR 1.61, p = 0.002, CI 1.18–2.18) added to model prediction (OR compared to Group 7).
In a multivariable analysis investigating variables associated with all-cause readmission during the 12-week follow-up period, a history of hypertension, LVSD, and aortic stenosis were identified in 25 cross validations using p < 0.05 for model selection. In the logistic regression, only Group 6 added to the model with OR of 1.69 (p < 0.001, CI 1.34–2.12) compared to Group 7. However, the area under the ROC curve was only 0.55. When p < 0.1 was used to select variables from 25 cross validations, a history of infection, a history of valve replacement, and mitral regurgitation were identified that improved the ROC to 0.57 and again, only Group 6 added to the logistic regression model (OR of 1.77, p < 0.001, CI 1.40–2.24).
Discussion
EHFS-1 was designed to investigate the overall burden of heart failure from a hospital perspective and not just a narrowly defined group of patients admitted with heart failure as a primary diagnosis and managed by cardiologists [3, 21]. The survey emphasizes the heterogeneity of patients hospitalized with suspected HF [22, 23]. Fewer than half of patients presented with severe breathlessness at rest and yet this has been the main focus of trials of AHF until now. Patients presenting primarily with increasing oedema appear to be a common, but neglected group of patients [24]; recent post-hoc analyses suggests that such patients might account for the response observed to agents for AHF such as serelaxin [25].
It is now fashionable to highlight the lack of progress in the treatment of AHF, as opposed to the huge progress made in the last 25 years for chronic HF [2, 26,27,28]. Unfortunately, the only Class 1, level of evidence A recommendation for the management of AHF in the ESC 2012 guidelines is thrombo-embolism prophylaxis, but it is reduced to Class 1, level of evidence B in ESC 2016 acute and chronic HF guidelines [29, 30]. In the most recent ESC guidelines (2016), no treatment was given a Class 1, level of evidence A recommendation for the management of AHF. There are many possible reasons for lack of progress in AHF [31]. The interventions studied may be truly ineffective or study design may have been inadequate [32, 33]. However, the heterogeneity of the patient population probably plays a major role. More precise patient selection, timing of intervention and targeting of therapy in clinical trials could reap large dividends [34]. More precise targeting does not necessarily mean more restrictive inclusion criteria. For instance, if congestion and peripheral oedema, which usually develops over several weeks, is the primary treatment target then there is little point in trying to enrol patients within a few hours of admission, which is logistically difficult from a research perspective and greatly reduces recruitment. Peripheral oedema often persists for many days after initiating treatment, allowing time for a new intervention to be introduced and its effects to become apparent. However, for patients with severe breathlessness due to pulmonary oedema, the onset is often abrupt; delaying intervention even for a few minutes may not be acceptable, and symptoms may have largely resolved within a few hours [2, 35]. There is only a small window of opportunity to show that a new intervention accelerates the improvement in symptoms. Whether health services would pay for a treatment that shortens the time to symptom relief by a few minutes is uncertain. Short-term treatments have not yet been shown to reduce the risk of longer term relapse or death.
Historically, the spectacular success in managing acute myocardial infarction (MI) was made possible by the development of coronary care units and the segregation of patients into ST elevation MI (STEMI) and non-ST elevation MI (NSTEMI) [36,37,38]. The same may be true for AHF not only in terms of their presentation, but also their underlying left ventricular phenotype and atrial rhythm and where they are managed [30, 39]. Most trials of AHF have enrolled a mixture of patients presenting with severe acute-onset breathlessness at rest (pulmonary oedema) and others with sub-acute worsening of peripheral congestion who are not breathless sitting upright at rest, but have orthopnoea and are breathless on minor exertion. The median time to enrolment in trials of AHF, with one exception, has never been less than 6 h, by which time most patients with acute pulmonary oedema have responded to a combination of diuretics and oxygen and have only residual symptoms [2, 40]. The one exception is the 3CPO study that enrolled patients with acute pulmonary oedema with heart and respiratory rates of 114 and 33, respectively, within a few hours of presentation [41].
Not unsurprisingly, patients presenting with cardiogenic shock, VT/VF, and ACS had a much worse in-hospital prognosis, but subsequent to discharge their prognosis, both in terms of readmissions and death, was rather similar to patients with other presentations. Clearly, these patients require urgent measures to correct the haemodynamic disturbance and to limit myocardial damage at the time of presentation. The short-term prognosis of patients with AHF reported in epidemiological studies will depend greatly on whether such patients are included. HF as a secondary, rather than primary, diagnosis may have a much worse short-term outcome, but few trials target such patients [18]. These findings highlight that HF is not a distinct diagnosis, but rather, a collection of clinical conditions with a great variety of underlying causes and clinical features, which fall under one umbrella term that is generally associated with a poor outcome. Targeting the therapeutic approach more accurately at specific patient subsets, presentations, aetiologies, and pathophysiologies and precipitating factors may yield greater benefit than a more generalised approach as is usual in current clinical trials. However, post-discharge outcome was rather similar regardless of presentation, even when it was unclear whether HF had precipitated or complicated the admission. Clinical trials often exclude patients who do not fulfil robust criteria for a diagnosis of HF but who, nonetheless, almost certainly have this diagnosis and also have a poor prognosis for whom either further investigation and better treatment is required [42].
Patient heterogeneity amongst patients presenting with AHF is a fundamental problem when designing and performing clinical trials. More accurate patient characterization for right treatment on right target is most crucial for success in clinical step for designs of new clinical trials and clinical practice. Clinical presentation, precipitating factors, underlying cardiac phenotype and aetiology of cardiac dysfunction might each be used to classify patients with the AHF and select them for trials. These episodes are main areas to be considered for better categorization of these patients. Preferably, these areas should be clearly identified before randomization to a new therapy. For example, patients presenting with pulmonary oedema due to ACS are likely to benefit from different treatments from those presenting with severe peripheral oedema and atrial fibrillation. Accurate classification requires a basic set of information including a medical history and physical examination, haematology and biochemistry profile, oxygen saturation, chest X-ray, an electrocardiogram, and an echocardiogram. However, urgent access to echocardiography is often problematic due to a lack of skilled personnel available either at unsociable hours or available on medical wards within a few hours or sometimes even days, if at all. Up skilling the medical workforce to provide at least basic echocardiography quickly and routinely should improve the management.
Limitations
EHFS-1 was conducted in 2000-01, but, until now, there have been no innovations in therapy for AHF and recent trials suggest that the 12 week mortality of AHF has changed little in the past 15 years [16, 43, 44]. We developed mutually exclusive categories of patients but of course; in reality, some patients will belong to more than one class. However, in most such clinical situations, one presentation dominates. Attempting to avoid falling into the trap of capturing data on only narrowly defined ‘cardiological’ heart failure may have caused some confusion and inconsistent answers for our international group of investigators, especially for patients who were taking loop diuretics, but who had not been diagnosed with heart failure. However, it is impossible to assess the quality of care with respect to investigation if only patients who already have a definitive diagnosis of heart failure are included. Many patients hospitalized with a diagnosis or features suspicious of HF were admitted primarily for another reason. Most had features to suggest that they did indeed have HF and these patients had a high morbidity and mortality subsequent to discharge. This group of patients has lower in-patient mortality but only slightly lower rates of readmission and death subsequent to discharge compared to other groups, although their rates of readmission for worsening heart failure and of cardiovascular deaths were substantially lower.
A major limitation of this survey was the failure to specifically ask about peripheral oedema. We assume that when peripheral oedema was the major presentation, these will have been classified as ‘other’. However, many patients presenting with oedema will have had breathlessness on mild exertion or even at rest and may have been classified as presenting with breathlessness.
Conclusion
Acute heart failure is a collection of syndromes with different clinical presentations, precipitating factors, underlying aetiology, and pathophysiology that may affect the clinical course, prognosis, and response to treatment. A common feature of AHF is poor outcomes and the unmet clinical need is still great. Focussing on specific presentations, such as acute pulmonary oedema, worsening peripheral oedema, or rapid AF, that may differ in their pathophysiology and treatment goals might succeed, where the current efforts have so far failed.
References
Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA et al (2008) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 10(10):933–989
McDonagh TA, Komajda M, Maggioni AP, Zannad F, Gheorghiade M, Metra M et al (2011) Clinical trials in acute heart failure: simpler solutions to complex problems. Consensus document arising from a European Society of Cardiology cardiovascular round-table think tank on acute heart failure, 12 May 2009. Eur J Heart Fail 13(12):1253–1260
Llorens P, Javaloyes P, Martin-Sanchez FJ, Jacob J, Herrero-Puente P, Gil V et al (2018) Time trends in characteristics, clinical course, and outcomes of 13,791 patients with acute heart failure. Clin Res Cardiol. https://doi.org/10.1007/s00392-018-1261-z
Shoaib A, Mabote T, Zuhair M, Kassianides X, Cleland J (2013) Acute heart failure (suspected or confirmed): initial diagnosis and subsequent evaluation with traditional and novel technologies. World J Cardiovasc Dis 3(3):290–300
Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL (2017) Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol 106(7):533–541
Clark AL, Cleland JG (2013) Causes and treatment of oedema in patients with heart failure. Nat Rev Cardiol 10(3):156–170
Miro O, Gil V, Xipell C, Sanchez C, Aguilo S, Martin-Sanchez FJ et al (2017) IMPROV-ED study: outcomes after discharge for an episode of acute-decompensated heart failure and comparison between patients discharged from the emergency department and hospital wards. Clin Res Cardiol 106(5):369–378
Allen LA, Hernandez AF, O’Connor CM, Felker GM (2009) End points for clinical trials in acute heart failure syndromes. J Am Coll Cardiol 53(24):2248–2258
Zanolla L, Zardini P (2003) Selection of endpoints for heart failure clinical trials. Eur J Heart Fail 5(6):717–723
Tamargo J, Rosano GMC, Delpón E, Ruilope L, López-Sendón J (2017) Pharmacological reasons that may explain why randomized clinical trials have failed in acute heart failure syndromes. Int J Cardiol 233:1–11
Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC et al (2003) The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J 24(5):442–463
Shiraishi Y, Nagai T, Kohsaka S, Goda A, Nagatomo Y, Mizuno A et al (2018) Outcome of hospitalised heart failure in Japan and the United Kingdom stratified by plasma N-terminal pro-B-type natriuretic peptide. Clin Res Cardiol. https://doi.org/10.1007/s00392-018-1283-6
Cleland JG, Swedberg K, Cohen-Solal A, Cosin-Aguilar J, Dietz R, Follath F et al (2000) The Euro Heart Failure Survey of the EUROHEART survey programme. A survey on the quality of care among patients with heart failure in Europe. The Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The Medicines Evaluation Group Centre for Health Economics University of York. Eur J Heart Fail 2(2):123–132
Sauerbrei W (1999) The use of resampling methods to simplify regression models in medical statistics. J R Stat Soc Ser C Appl Stat 48(3):313–329
Ingle L, Rigby AS, Carroll S, Butterly R, King RF, Cooke CB et al (2007) Prognostic value of the 6 min walk test and self-perceived symptom severity in older patients with chronic heart failure. Eur Heart J 28(5):560–568
Cleland JG, Chiswell K, Teerlink JR, Stevens S, Fiuzat M, Givertz MM et al (2014) Predictors of postdischarge outcomes from information acquired shortly after admission for acute heart failure: a report from the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Study. Circ Heart Fail 7(1):76–87
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148(3):839–843
Shoaib A, Farag M, Nasir M, John J, Gupta S, Pellicori P et al (2016) Is the diagnostic coding position of acute heart failure related to mortality? A report from the Euro Heart Failure Survey-1. Eur J Heart Fail 18(5):556–563
Zucchini W (2000) An Introduction to Model Selection. J Math Psychol 44(1):41–61
Raftery AE (1995) Bayesian model selection in social research. Sociol Methodol 25:111–163
Miro O, Gil VI, Martin-Sanchez FJ, Jacob J, Herrero P, Alquezar A et al (2018) Short-term outcomes of heart failure patients with reduced and preserved ejection fraction after acute decompensation according to the final destination after emergency department care. Clin Res Cardiol 107(8):698–710
Felker GM, Adams KF, Konstam MA, O’Connor CM, Gheorghiade M (2003) The problem of decompensated heart failure: nomenclature, classification, and risk stratification. Am Heart J 145(2):S18–S25
Gheorghiade M, Zannad F, Sopko G, Klein L, Piña IL, Konstam MA et al (2005) Acute heart failure syndromes current state framework for future research. Circulation 112(25):3958–3968
Shoaib A, Waleed M, Khan S, Raza A, Zuhair M, Kassianides X et al (2014) Breathlessness at rest is not the dominant presentation of patients admitted with heart failure. Eur J Heart Fail 16:1283–1291
Gimpelewicz C, Metra M, Cleland JGF, Szecsody P, Chang Wun CC, Boer-Martins L et al (2017) Effects of serelaxin on the outcome of patients with or without substantial peripheral edema: a subgroup analysis from the RELAX-AHF trial. Am Heart J 190:113–122
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L et al (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352(15):1539–1549
CONSENSUS Trial Study Group (1987) Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 316(23):1429–1435
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A et al (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341(10):709–717
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K et al (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33(14):1787–1847
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ et al (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (English ed) 69(12):1167
Tamargo J, Rosano GMC, Delpón E, Ruilope L, López-Sendón J (2016) Pharmacological reasons that may explain why randomized clinical trials have failed in acute heart failure syndromes. Int J Cardiol 233:1–11
Teichman SL, Maisel AS, Storrow AB (2015) Challenges in acute heart failure clinical management: optimizing care despite incomplete evidence and imperfect drugs. Crit Pathw Cardiol 14(1):12–24
Pang PS, Givertz MM (2013) The challenge of drug development in acute heart failure. Balancing mechanisms, targeting patients, and gambling on outcomes. JCHF 1(5):442–444
Palazzuoli A, Ruocco G, Beltrami M, Nuti R, Cleland JG (2018) Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin Res Cardiol 107(7):586–596
Shoaib A, Raza A, Waleed M, Djahit A, Kassianides X et al (2013) Presenting symptoms and changes in heart and respiratory rate and blood pressure in the first 24 hours after admission in patients admitted with heart failure. ESC Heart Fail 16:1283–1291
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD (2012) Third universal definition of myocardial infarction. Circulation 126(16):2020–2035
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F et al (2016) 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 37(3):267–315
Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA et al (2012) ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33(20):2569–2619
Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG et al (2014) Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet 384(9961):2235–2243
Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S et al (2010) The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J 31(7):832–841
Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J (2008) Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 359(2):142–151
Riedel O, Ohlmeier C, Enders D, Elsasser A, Vizcaya D, Michel A et al (2018) The contribution of comorbidities to mortality in hospitalized patients with heart failure. Clin Res Cardiol 107(6):487–497
Passantino A, Monitillo F, Iacoviello M, Scrutinio D (2015) Predicting mortality in patients with acute heart failure: role of risk scores. World J Cardiol 7(12):902–911
O’Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M et al (2008) Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 156(4):662–673
Author information
Authors and Affiliations
Contributions
AS: conceived study idea, statistical analysis, and writing. MF: intellectual input and manuscript writing. JN: intellectual input, manuscript writing, and reviewing. AR: review and supervision of statistical analysis. AP: intellectual input and manuscript writing. MR: intellectual input, manuscript writing, and making figure. CSK: intellectual input and manuscript writing. RP: intellectual input and manuscript writing. ALC: supervisor, intellectual input, and manuscript writing. MK: conceived study idea, intellectual input, and manuscript writing. JGFC: conceived study idea, intellectual input, statistical analysis planning, and manuscript writing and reviewing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shoaib, A., Farag, M., Nolan, J. et al. Mode of presentation and mortality amongst patients hospitalized with heart failure? A report from the First Euro Heart Failure Survey. Clin Res Cardiol 108, 510–519 (2019). https://doi.org/10.1007/s00392-018-1380-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1380-6