Abstract
Objectives
Parenteral prostanoids infused via external pumps are well-established pulmonary arterial hypertension (PAH) treatments. However, local side-effects and systemic infections restrict their use. The purpose of this study was to investigate the safety of a fully implantable treprostinil infusion pump (LENUS Pro®) in patients with PAH.
Methods
Thirty patients with PAH undergoing pump implantation (with stable PAH therapy for ≥3 weeks pre-implantation) were included in this prospective, multicenter, observational study (NCT01979822). Primary endpoints were predefined adverse events (AEs) during implantation, in-hospital and/or during 6-month follow-up. Refill-related AEs were a secondary endpoint.
Results
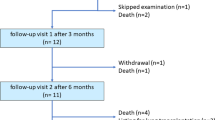
Twenty-nine patients completed 6-month follow-up (one underwent lung transplantation). During implantation, one pneumothorax (not requiring drainage) occurred. Four patients had an in-hospital AE (including one catheter revision). During 6-month follow-up, AEs were most frequent at the first refill (10); the most common AE was seroma around the pump. No infections occurred. One pump required replacement because of a defective septum caused by use of a non-approved refill needle (associated with extravasation). Apart from the extravasation, no refill-related AEs were recorded. Post hoc efficacy analyses showed significant improvements in functional class [number in functional class I/II/III/IV: 0/5/21/2 (baseline) versus 3/8/17/0 (6 months); p = 0.012] and 6-min walk distance (mean ± standard deviation: 407 ± 122 m versus 445 ± 127 m; n = 17; p = 0.014).
Conclusions
This study supports use of a fully implantable treprostinil infusion pump in patients with PAH requiring parenteral prostanoids. Refills should be performed by specialized healthcare professionals at patients’ homes or at experienced centers using approved equipment.


Similar content being viewed by others
References
McLaughlin VV, Shah SJ, Souza R, Humbert M (2015) Management of pulmonary arterial hypertension. J Am Coll Cardiol 65:1976–1997. doi:10.1016/j.jacc.2015.03.540
Frumkin LR (2012) The pharmacological treatment of pulmonary arterial hypertension. Pharmacol Rev 64:583–620. doi:10.1124/pr.111.005587
Galie N, Humbert M, Vachiery JL et al (2015) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 46:903–975. doi:10.1183/13993003.01032-2015
McLaughlin VV, Archer SL, Badesch DB et al (2009) ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 53:1573–1619. doi:10.1016/j.jacc.2009.01.004
Taichman DB, Ornelas J, Chung L et al (2014) Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest 146:449–475. doi:10.1378/chest.14-0793
Barst RJ, Rubin LJ, Long WA et al (1996) A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 334:296–302
Doran AK, Ivy DD, Barst RJ et al (2008) Guidelines for the prevention of central venous catheter-related blood stream infections with prostanoid therapy for pulmonary arterial hypertension. Int J Clin Pract Suppl 160:5–9. doi:10.1111/j.1742-1241.2008.01811.x
Laliberte K, Arneson C, Jeffs R, Hunt T, Wade M (2004) Pharmacokinetics and steady-state bioequivalence of treprostinil sodium (Remodulin) administered by the intravenous and subcutaneous route to normal volunteers. J Cardiovasc Pharmacol 44:209–214
Simonneau G, Barst RJ, Galie N et al (2002) Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 165:800–804. doi:10.1164/ajrccm.165.6.2106079
Hiremath J, Thanikachalam S, Parikh K et al (2010) Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant 29:137–149. doi:10.1016/j.healun.2009.09.005
Kitterman N, Poms A, Miller DP, Lombardi S, Farber HW, Barst RJ (2012) Bloodstream infections in patients with pulmonary arterial hypertension treated with intravenous prostanoids: insights from the REVEAL REGISTRY(R). Mayo Clin Proc 87:825–834. doi:10.1016/j.mayocp.2012.05.014
Rich JD, Glassner C, Wade M et al (2012) The effect of diluent pH on bloodstream infection rates in patients receiving IV treprostinil for pulmonary arterial hypertension. Chest 141:36–42. doi:10.1378/chest.11-0245
Kingman MS, Tankersley MA, Lombardi S et al (2010) Prostacyclin administration errors in pulmonary arterial hypertension patients admitted to hospitals in the United States: a national survey. J Heart Lung Transplant 29:841–846. doi:10.1016/j.healun.2010.03.008
Dario A, Scamoni C, Picano M, Fortini G, Cuffari S, Tomei G (2005) The infection risk of intrathecal drug infusion pumps after multiple refill procedures. Neuromodulation 8:36–39. doi:10.1111/j.1094-7159.2005.05218.x
Naumann C, Erdine S, Koulousakis A, Van Buyten JP, Schuchard M (1999) Drug adverse events and system complications of intrathecal opioid delivery for pain: origins, detection, manifestations, and management. Neuromodulation 2:92–107. doi:10.1046/j.1525-1403.1999.00092.x
Bourge RC, Waxman AB, Gomberg-Maitland M et al (2016) Treprostinil administered to treat pulmonary arterial hypertension using a fully implantable programmable intravascular delivery system: results of the DelIVery for PAH Trial. Chest 150:27–34. doi:10.1016/j.chest.2015.11.005
Desole S, Velik-Salchner C, Fraedrich G, Ewert R, Kahler CM (2012) Subcutaneous implantation of a new intravenous pump system for prostacyclin treatment in patients with pulmonary arterial hypertension. Heart Lung 41:599–605. doi:10.1016/j.hrtlng.2012.07.001
Ewert R, Halank M, Bruch L, Ghofrani HA (2012) A case series of patients with severe pulmonary hypertension receiving an implantable pump for intravenous prostanoid therapy. Am J Respir Crit Care Med 186:1196–1198. doi:10.1164/ajrccm.186.11.1196
Steringer-Mascherbauer R, Eder V, Huber C et al (2012) First long-term experience with intravenous treprostinil administered by the implantable infusion pump LenusPro. A single-center pilot study. Eur Respir J 40:165S (abstract)
Richter MJ, Ewert R, Warnke C et al. (2017) Procedural safety of a fully implantable intravenous prostanoid pump for pulmonary hypertension. Clin Res Cardiol 106(3):174–182. doi:10.1007/s00392-016-1037-2
Hoeper MM, Gall H, Seyfarth HJ et al (2009) Long-term outcome with intravenous iloprost in pulmonary arterial hypertension. Eur Respir J 34:132–137. doi:10.1183/09031936.00130408
Mudan S, Giakoustidis A, Morrison D et al (2015) 1000 Port-A-Cath (R) placements by subclavian vein approach: single surgeon experience. World J Surg 39:328–334. doi:10.1007/s00268-014-2802-x
Plumhans C, Mahnken AH, Ocklenburg C et al (2011) Jugular versus subclavian totally implantable access ports: catheter position, complications and intrainterventional pain perception. Eur J Radiol 79:338–342. doi:10.1016/j.ejrad.2009.12.010
Meyer S, McLaughlin VV, Seyfarth HJ et al (2013) Outcomes of noncardiac, nonobstetric surgery in patients with PAH: an international prospective survey. Eur Respir J 41:1302–1307. doi:10.1183/09031936.00089212
Ramakrishna G, Sprung J, Ravi BS, Chandrasekaran K, McGoon MD (2005) Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol 45:1691–1699. doi:10.1016/j.jacc.2005.02.055
Price LC, Montani D, Jais X et al (2010) Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J 35:1294–1302. doi:10.1183/09031936.00113009
Kaw R, Pasupuleti V, Deshpande A, Hamieh T, Walker E, Minai OA (2011) Pulmonary hypertension: an important predictor of outcomes in patients undergoing non-cardiac surgery. Respir Med 105:619–624. doi:10.1016/j.rmed.2010.12.006
Lai HC, Lai HC, Wang KY, Lee WL, Ting CT, Liu TJ (2007) Severe pulmonary hypertension complicates postoperative outcome of non-cardiac surgery. Br J Anaesth 99:184–190. doi:10.1093/bja/aem126
Ray S, Stacey R, Imrie M, Filshie J (1996) A review of 560 Hickman catheter insertions. Anaesthesia 51:981–985
Baskin JL, Pui CH, Reiss U et al (2009) Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 374:159–169. doi:10.1016/S0140-6736(09)60220-8
Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM (2015) Complications of central venous access devices: a systematic review. Pediatrics 136:e1331–e1344. doi:10.1542/peds.2015-1507
Oudiz RJ, Widlitz A, Beckmann XJ et al (2004) Micrococcus-associated central venous catheter infection in patients with pulmonary arterial hypertension. Chest 126:90–94. doi:10.1378/chest.126.1.90
Acknowledgements
We would like to thank all participating pulmonary hypertension centers, the surgeons who implanted the pump, and all dedicated support staff for taking care of the patients’ LENUS Pro® refills. In addition, we would like to thank Dr. Anne Obst (University of Greifswald) for statistical assistance. This study was supported by the University of Greifswald, Germany. Editorial assistance was provided by Dr. Claire Mulligan (Beacon Medical Communications Ltd, Brighton, UK) funded by OMT GmbH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Ewert has received speaker fees and fees for participation in advisory boards from United Therapeutics/OMT, Pfizer, GlaxoSmithKline (GSK), Actelion, Novartis, Bayer HealthCare, and Encysive/Pfizer, and grants from Actelion and Boehringer Ingelheim. Dr. Steringer-Mascherbauer has received unrestricted grants from Actelion, GSK, AOP and Bayer, and fees for participation in advisory boards from Actelion, MSD and United Therapeutics. Dr. Grünig has received speaker fees from Bayer, Actelion, Pfizer, and GSK; fees for participation in advisory boards from Bayer, Pfizer, GSK, and Actelion; and industry-sponsored grants from Bayer HealthCare and Actelion. Dr. Lange has received speaker fees, honoraria for consultations, and research funding from Actelion, AOP Orphan Pharmaceuticals, Bayer, GSK, Novartis, Pfizer, and United Therapeutics. Dr. Opitz’s institution has received speaker fees and honoraria for consultations from Actelion, Bayer, GSK and Pfizer. Dr. Warnke has received speaker fees from United Therapeutics/OMT. Dr. Richter has received support from United Therapeutics and Bayer Pharma AG, and speaker fees from Actelion, Mundipharma, Roche, and United Therapeutics/OMT. Dr. Ghofrani has received consultancy fees from Bayer, Actelion, Pfizer, Merck, GSK, and Novartis; fees for participation in advisory boards from Bayer, Pfizer, GSK, Actelion, and Takeda; lecture fees from Bayer HealthCare, GSK, Actelion, and Encysive/Pfizer; industry-sponsored grants from Bayer HealthCare, Aires, Encysive/Pfizer, and Novartis; and sponsored grants from the German Research Foundation, Excellence Cluster Cardiopulmonary Research, and the German Ministry for Education and Research.
Ethical standards
The study was approved by the leading ethics committee of the University of Greifswald (BB 109/12, Amendment 109/12a), and local ethics committees of the recruiting centers if applicable; the study was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients gave written informed consent prior to their inclusion in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ewert, R., Richter, M.J., Steringer-Mascherbauer, R. et al. Intravenous treprostinil infusion via a fully implantable pump for pulmonary arterial hypertension. Clin Res Cardiol 106, 776–783 (2017). https://doi.org/10.1007/s00392-017-1114-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-017-1114-1