Abstract
Objective
We tested whether bedside testing for H-FABP is, alone or integrated in combination models, useful for rapid risk stratification of non-high-risk PE.
Methods
We prospectively studied 136 normotensive patients with confirmed PE. H-FABP was determined using a qualitative bedside-test showing a positive result for plasma concentration >7 ng/ml.
Results
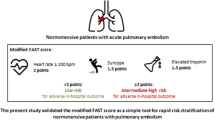
Overall, 11 patients (8.1 %) had an adverse 30-day outcome. Of 58 patients (42.6 %) with a positive H-FABP bedside-test, 9 (15.5 %) had an unfavourable course compared to 2 of 78 patients (2.6 %) with a negative test result (p = 0.009). Logistic regression analysis indicated a sevenfold increased risk for an adverse outcome (95 % CI, 1.45–33.67; p = 0.016) for patients with a positive H-FABP bedside-test. Additive prognostic information were obtained by a novel score including the H-FABP bedside-test (1.5 points), tachycardia (2 points), and syncope (1.5 points) (OR 11.57 [2.38–56.24]; p = 0.002 for ≥3 points). Increasing points were associated with a continuous exponential increase in the rate of an adverse 30-day outcome (0 % for patients with 0 points and 44.4 % for ≥5 points). Notably, this simple score provided similar prognostic value as the combination of the H-FABP bedside-test with echocardiographic signs of right ventricular dysfunction (OR 12.73 [2.51–64.43]; p = 0.002).
Conclusions
Bedside testing for H-FABP appears a useful tool for immediate risk stratification of non-high-risk patients with acute PE, who may be at increased risk of an adverse outcome, in particular if integrated in a novel score without the need of echocardiographic examination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
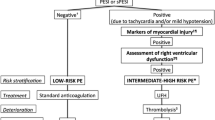
Acute pulmonary embolism (PE) is a frequent and life-threatening disease. As proposed by current guidelines [1, 2], risk stratification is based on clinical assessment of haemodynamic instability in order to identify patients who are at high risk of early death or life-threatening complications. Furthermore, laboratory biomarkers and imaging procedures can be used for the assessment of right ventricular (RV) dysfunction and injury and thus for classifying non-high-risk patients into an intermediate-risk and a low-risk subgroup. Currently, the most widely used laboratory markers of myocardial (RV) dysfunction and injury, natriuretic peptides [3] and cardiac troponins [4, 5], are characterised by low specificity and positive predictive values and do not, by themselves, justify more aggressive treatment regimens [6]. Therefore, in the past years, strategies for optimising risk stratification of normotensive patients with acute PE have focused on (1) combination models integrating information from laboratory markers and imaging procedures [7–9]; (2) clinical scores of PE severity [10–14]; and (3) novel, promising biomarkers [9, 15].
Recently, we and others demonstrated that heart-type fatty acid-binding protein (H-FABP), an early and sensitive marker of myocardial injury with favourable release kinetics [16], is of prognostic value in patients with acute PE and improves risk stratification of both, unselected [17, 18] and haemodynamic stable patients [19]. In fact, H-FABP appeared superior to cardiac troponins and natriuretic peptides for predicting an adverse outcome [19]. In those studies, H-FABP concentrations were determined using quantitative solid-phase enzyme-linked immunoadsorbent assays based on the sandwich principle (ELISAs) which are currently not available for application in clinical routine. Therefore, a point-of-care test for H-FABP was developed that allows rapid (within 20 min) qualitative determination of H-FABP concentrations in full blood or plasma [20]. This assay emerged as a reliable test for the early diagnosis of acute myocardial infarction [21, 22]. In intermediate-risk patients with acute PE, positive test results indicating H-FABP plasma concentrations above 7 ng/ml were associated with impaired RV function [23].
The aim of the present study was to determine whether bedside testing of H-FABP is—alone or integrated in combination models with other predictors of an adverse outcome—capable of accelerating and simplifying risk stratification of non-high-risk PE.
Methods
Patient population and study design
Consecutive patients who were diagnosed with acute symptomatic PE were prospectively studied at the University of Göttingen between October 2005 and April 2009. For inclusion in the study, diagnosis of PE had to be confirmed by an imaging procedure (contrast-enhanced multidetector computed tomography, ventilation–perfusion lung scan, or pulmonary angiography; or by echocardiography showing the presence of mobile thrombi in the right atrium or ventricle, or in the proximal portions of the pulmonary artery) based on the diagnostic algorithms proposed by recent guidelines [1, 2] and those existing before 2008 [24, 25]. Patients were excluded from the study if they met at least one of the following criteria: (1) haemodynamic instability at presentation, defined as the presence of the following: need for cardiopulmonary resuscitation, systolic blood pressure <90 mmHg or drop of systolic blood pressure by ≥40 mmHg for ≥15 min with signs of end-organ hypoperfusion, or need for catecholamine administration to maintain adequate organ perfusion and a systolic blood pressure ≥90 mm Hg; (2) PE being an accidental finding obtained during diagnostic workup for another suspected disease; and (3) denial of consent or withdrawal of previously given consent for participation in the study.
According to the study protocol, and as described previously [15, 19], complete data on baseline clinical, haemodynamic, and laboratory parameters were obtained using a standardised questionnaire. Treatment decisions were made by the physicians caring for the patient according to the mentioned guidelines and not influenced by the study protocol. Study results were not communicated to the clinicians and thus not used to guide the patient’s management or to monitor the effects of treatment during the hospital stay or at any time during the 30-day follow-up period.
A transthoracic echocardiogram was strongly recommended by the study protocol. Right ventricular (RV) dysfunction was defined as dilatation of the right ventricle (end-diastolic diameter >30 mm from the parasternal view, or a right/left ventricle diameter ratio ≥1.0 from the subcostal or apical view) combined with absence of inspiratory collapse of the inferior vena cava, in the absence of left ventricular or mitral valve disease [15, 19, 26].
Thirty-day clinical follow-up data were obtained from all patients included in the study. An adverse 30-day outcome was defined as death from any cause or at least one of the following major complications [15, 19]: (1) need for intravenous catecholamine administration (except for dopamine at a rate of ≤5 μg/kg/min) to maintain adequate tissue perfusion and prevent or treat cardiogenic shock; (2) endotracheal intubation; and (3) cardiopulmonary resuscitation. The causes of death were adjudicated by two of the authors (M.L. and C.D.) by reviewing the patients’ medical records and the results of autopsy if performed.
The study protocol was approved by the Ethical Committee of the University of Göttingen.
Laboratory parameters and biomarker testing
Venous plasma samples were collected on admission and immediately stored at −80 °C. Samples were later analysed in batches after a single thaw.
Qualitative plasma concentrations of H-FABP were determined using a rapid chromatographic immunoassay (“bedside-test”; CardioDetect® lab, Rennessens GmbH, Berlin, Germany), as previously described [20, 21]. The test, which is approved for plasma, serum and whole blood samples, shows a “positive” test result for H-FABP plasma concentrations above 7 ng/ml, and test results are available within 20 min.
Routine laboratory parameter measurements including the conventional assays for cardiac troponin T (cTnT) and N-terminal pro-brain natriuretic peptide (NT-proBNP) (quantitative electrochemiluminescence immunoassays (Elecsys 1010/2010 analyzer, Roche Diagnostics, Mannheim, Germany) were performed at the Department of Clinical Chemistry of the University of Göttingen. For NT-proBNP, a concentration of 1,000 pg/ml was defined as cut-off value for distinguishing between normal and elevated plasma levels [7, 15], and for cTnT a concentration of 0.03 ng/ml as specified by the manufacturer. The glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease (MDRD) study equation; renal insufficiency was defined as GFR <60 ml/min/1.73 m2 body-surface area.
Statistical analysis
Continuous variables were found not to follow a normal distribution as tested with the modified Kolmogorov–Smirnov test (Lilliefors test). They were therefore expressed as medians with corresponding 25th and 75th percentiles and compared using the unpaired Mann–Whitney U test. Categorical variables were compared using Fisher’s exact test or Chi2 test, as appropriate. Receiver operating characteristic (ROC) analysis was used to determine the area under the curve (AUC) of baseline biomarker concentrations and the novel score with regard to an adverse 30-day outcome. Furthermore, ROC analysis was used for defining the optimal cut-off value of the novel score. Sensitivity, specificity, and the positive and negative predictive value of elevated biomarker levels and the novel score were calculated. The prognostic relevance of a positive H-FABP bedside-test, elevated biomarker concentrations, and other baseline parameters (as listed in Table 1) as well as of the combination models with regard to 30-day outcome was estimated using logistic regression analysis. Odds ratios (OR) and the corresponding 95 % confidence intervals (CI) were calculated.
All tests were two-sided and used a significance level of 0.05. Analyses were performed using the PASW software (version 18.0, Chicago, Illinois, USA).
Results
Baseline clinical and laboratory findings
Overall, 136 normotensive patients with acute PE were included in the study (derivation cohort). The baseline clinical characteristics of the study patients are summarised in Table 1. Diagnosis of PE was confirmed by contrast-enhanced multidetector computed tomography (n = 120, 88.2 %), ventilation–perfusion lung scan (n = 13, 9.6 %), or pulmonary angiography (n = 1; 0.7 %). In two patients (1.5 %), the diagnosis of PE was established by echocardiographic criteria (as explained in the “Methods”). Overall, a transthoracic echocardiogram was performed in 102 patients (75.0 %); of these, 48 patients (47.1 %) were diagnosed with RV dysfunction.
On admission, the H-FABP bedside-test was positive in 58 patients (42.6 %). As shown in Table 1, patients with a positive H-FABP bedside-test were older and more frequently diagnosed with congestive heart failure and renal insufficiency. These patients were more likely to present with syncope but less likely to present with chest pain. Cardiac TnT levels ranged from 0.01 to 0.34 ng/ml with a median value of 0.01 (25th to 75th percentile, 0.01–0.05) ng/ml, and 47 patients (35.6 %) had cTnT levels above the cut-off value of 0.03 ng/ml. NT-proBNP levels ranged from 10 to 32,156 pg/ml with a median value of 766 (126–2,371) pg/ml, and 64 patients (47.4 %) had NT-proBNP levels above the cut-off value of 1,000 pg/ml. Both biomarkers were higher in patients with a positive H-FABP bedside-test (p < 0.001 each).
H-FABP bedside testing for predicting early outcome after acute PE
During the acute phase of PE (first 30 days), 11 patients (8.1 %) had an adverse outcome. Overall, 7 patients (5.1 %) died; four deaths were due to PE and three to cancer as the underlying disease. Patients with an adverse 30-day outcome presented more often with a positive H-FABP bedside-test on admission compared to patients with a favourable course (81.8 vs. 39.2 %; p = 0.009). As shown in Table 2, a positive H-FABP bedside-test alone was associated with a prognostic sensitivity (82 %) and specificity (61 %). Overall, 15.5 % of the patients with a positive H-FABP bedside-test on admission died or developed life-threatening complications, while 2.6 % of those patients with a negative test had an adverse 30-day outcome. ROC analysis showed an AUC of 0.713 (95 % CI, 0.567–0.859) for the H-FABP bedside-test, compared to 0.654 (0.515–0.792) for cTnT and 0.639 (0.479–0.800) for NT-proBNP.
Univariable logistic regression analysis indicated a sevenfold increase in the risk of an adverse 30-day outcome (95 % CI, 1.45–33.67; p = 0.016) for patients with a positive H-FABP bedside-test. As shown in Table 3, besides a positive H-FABP bedside-test, tachycardia, evidence of RV dysfunction on echocardiography, and syncope were identified as being univariably correlated with a poor outcome, whereas elevation of the established biomarkers cTnT (p = 0.432) and NT-proBNP (p = 0.094) above their cut-off values did not appear to provide prognostic information.
Integration of the H-FABP bedside-test into combination models
We investigated whether the combination of the H-FABP bedside-test with other predictors of an adverse outcome might further improve risk stratification of acute PE. Indeed, the combination of the H-FABP bedside-test with evidence of RV dysfunction on echocardiography was associated with a 12.73-fold increase in the risk of an adverse 30-day outcome (2.51–64.43; p = 0.002), which appeared superior to the prognostic information provided by the H-FABP bedside-test alone (Table 3). Of the 102 patients with an echocardiographic examination on admission, 30 patients (29.4 %) had a positive H-FABP bedside-test and evidence of RV dysfunction on echocardiography and 8 of them (26.7 %) had an adverse 30-day outcome. On the other hand, two of 72 patients (2.8 %) with a negative H-FABP bedside-test and/or a normal echocardiogram died or had serious complications (p = 0.001). Overall, the combination model was associated with a prognostic sensitivity of 80 %, a specificity of 76 %, a positive predictive value (PPV) of 27 %, and a negative predictive value (NPV) of 97 %. In comparison, neither cTnT nor NT-proBNP improved the prognostic information provided by echocardiography alone (data not shown).
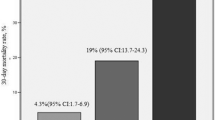
Since echocardiography may not be available on a round-the-clock basis in many hospitals, and in view of the fact that echocardiographic criteria for the detection of acute RV dysfunction are often vague and poorly standardised [26, 27], we tested whether comparable prognostic information could be obtained by an alternative combination model based on bedside parameters for rapid risk assessment. For this purpose, all clinical variables that were identified as univariable predictors of an adverse 30-day outcome (Table 3) were included in a score and the respective weight was obtained from the regression coefficient of the multivariable logistic regression analysis. Thus, a positive H-FABP bedside-test “weighted” 1.5 points; tachycardia, 2.0 points; and syncope, 1.5 points. By ROC analysis (AUC, 0.847 [0.746–0.949]) we identified an optimal cut-off value of 3.0 points for discriminating between patients with an adverse 30-day outcome and those with a favourable course. Of 44 patients (32.4 %) with a score of ≥3.0 points, 9 patients (20.5 %) developed complications or died during the first 30 days as opposed to only two of 92 patients (2.2 %) with a score of <3 points (p = 0.001). The dichotomised score was associated with a sensitivity of 82 %, a specificity of 72 %, a PPV of 20 %, and a NPV of 98 %. Using logistic regression analysis, a score of ≥3.0 points was associated with a nearly 12-fold increase in the risk of an adverse 30-day outcome (OR 11.57 [2.38–56.24]; p = 0.002). The prognostic relevance of the novel score remained unaffected if adjusted for age (data not shown). Notably, patients with a score ≥5.0 points had a 13.7-fold increase in the risk of an adverse 30-day outcome ([3.00–62.69]; p = 0.001) and a rate of an adverse 30-day outcome of 44.4 % (Fig. 1). Moreover, increasing points in the score were associated with a continuous exponential increase in the rate of an adverse 30-day outcome (Fig. 1; p < 0.001 for trend). Importantly, none of the 48 patients with a score of 0 points had an adverse outcome.
Discussion
Detection of an elevated risk amongst normotensive patients with acute PE remains challenging despite the large number of studies published in the last decade. As currently available biomarker assays share the requirement for time-consuming measurements, the development of bedside-tests for rapid determination of biomarker concentrations appears to be a promising approach. In the present study, we could demonstrate that bedside testing for H-FABP is a useful tool for immediate risk stratification of normotensive patients with acute PE. Besides the association of a positive test result with an adverse early outcome, the H-FABP bedside-test appeared to provide valuable prognostic information when integrated into a novel, “simple” clinical score also including tachycardia and syncope.
H-FABP is a promising biomarker of myocardial injury with favourable release kinetics [16]. Due to its small molecular size (15 kDa) and its cytoplasmatic location, H-FABP plasma concentrations rise as early as 30 min after the onset of myocardial ischemia, peak at 6–8 h, and return to normal within 24–30 h [28]. We and others previously demonstrated that H-FABP is of prognostic value in patients with acute PE and improves risk stratification of both unselected and normotensive patients [17–19]. However, widespread clinical use of H-FABP has been prevented by the need for a time-consuming solid-phase ELISA. A novel point-of-care test, the CardioDetect® lab assay, allows qualitative determination of H-FABP concentrations (positive vs. negative test result) within 20 min [20]. We could now demonstrate in 136 normotensive patients with acute PE that the H-FABP bedside-test emerged, besides tachycardia, evidence of RV dysfunction on echocardiography, and syncope, as predictor of an adverse early outcome.
Multimarker models integrating information obtained from echocardiography (evidence or exclusion of RV dysfunction) in combination with laboratory biomarkers (such as growth-differentiation factor-15 (GDF-15) [9], NT-proBNP [7], cTnT [8, 29]/hsTnT [15], or H-FABP [18, 19]) have been reported to improve risk stratification of acute PE. This could be confirmed in the present study, in which the combination of echocardiographic evidence of RV dysfunction with the H-FABP bedside-test predicted a 12.7-fold increase in the risk of an adverse 30-day outcome. However, it needs to be kept in mind that echocardiographic criteria for defining acute RV dysfunction are poorly standardised and may vary widely between hospitals, ultrasound laboratories, and examiners [26, 30]. Moreover, echocardiography may not be available outside the working hours, especially in smaller community hospitals. This problem might be expected to occur even more frequently in normotensive patients with acute PE, who are generally not considered “critically ill”. Therefore, we developed a “simple” clinical score derived from the significant predictors of an adverse 30-day outcome at univariable logistic regression (excluding echocardiographic information on RV function). This score included the H-FABP bedside-test (a positive result was assigned 1.5 points), tachycardia (heart rate above 100 beats/min on admission assigned 2 points), and syncope (presence of syncope assigned 1.5 points). The present score thus integrates weighed prognostic information from baseline clinical parameters which are easy to determine in clinical routine, in combination with a biomarker which can be measured by a point-of-care test within 20 min. We found that patients with a score above the calculated cut-off value of 3.0 points had a 12-fold increase in the risk of an adverse 30-day outcome. In fact, this was nearly identical with the prognostic information provided by the combination of echocardiography and the H-FABP bedside-test. Importantly, none of the 48 patients (35.3 %) with a score of 0 had an adverse outcome, while two (2.8 %) of the 72 patients with a negative H-FABP bedside-test and/or normal echocardiogram had an unfavourable course. Further, an increasing score was associated with a continuous exponential increase in the rate of an adverse 30-day outcome (Fig. 1). Therefore, calculation of the new score may offer a valuable and fast alternative for immediate risk assessment of normotensive patients with acute PE if echocardiography is not available. Pending external validation of our results, the novel score might simplify and accelerate risk stratification of PE patients in clinical routine in the future.
In the last years, a number of clinical prediction rules were developed for prognostic assessment of patients with acute PE. The pulmonary embolism severity index (PESI) [10] focuses on 11 different weighted patient characteristics such as comorbidities and baseline clinical parameters and allows stratification into five severity classes. Its simplified version (sPESI) [11] reduces the technical complexity of the original prediction rule by focusing on six equally weighted variables. However, the (s)PESI, as well as a score developed by Uresandi et al. [13], appears to be more suitable for the identification of low-risk patients than for patients with an elevated risk of an adverse outcome. The Geneva Score [14] focuses on six different variables such as comorbidities and haemodynamic parameters but also needs an ultrasound examination of the leg veins which may not be available in many hospitals. The PREP score [31] is based on five different weighted clinical, echocardiographic [RV/LV ratio), and biochemical variables (brain natriuretic peptide (BNP)] and allows stratification in five severity classes. Another recently proposed prognostic model consists of NT-proBNP, D-dimer concentrations, heart rate, and cancer with a total score range from 0 to 37 points [12]. The novel score which we developed in the present study is the first to integrate baseline clinical parameters and prognostic information obtained from a cardiac biomarker (in contrast to the (s)PESI which does not account for right ventricular dysfunction) without the need of a transthoracic echocardiogram (as in the PREP score) or ultrasound examination of the leg veins (as in the Geneva Score). Our score is characterised by low complexity, with only three differently weighed variables, and by the use of a bedside-test for fast and immediate risk stratification.
As the purpose of our study was to investigate the performance of the H-FABP bedside-test, we did not compare it with the quantitative solid-phase enzyme-linked immunoadsorbent assay (ELISA) for determination of H-FABP used in previous studies. Hence, our results may be limited regarding the universal use of H-FABP values in the novel score. However, findings from Boscheri et al. [23] indicate that the CardioDetect® lab assay performs as reliably as the H-FABP ELISA (HyCult biotechnology b.v., Uden, Netherlands). Another limitation is the missing of an external validation cohort. Thus, the novel score and the application of the bedside-test require external validation in normotensive patients with acute PE and we are hereby providing the base of further clinical studies. Moreover, the relatively small number of events in the acute phase of normotensive PE is a potential limitation of the present study.
In conclusion, we could demonstrate that the H-FABP bedside-test reliably identified an increased risk of an adverse early outcome in a derivation cohort of 136 normotensive patients with acute PE. Importantly, the integration of the bedside-test into a novel score also including two baseline clinical parameters, namely tachycardia and syncope, may offer a simple, readily available and fast approach to immediate risk assessment of PE, particularly if imaging of the right ventricle cannot be rapidly obtained.
References
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 123:1788–1830
Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D, Janssens U, Klepetko W, Mayer E, Remy-Jardin M, Bassand JP (2008) Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 29:2276–2315
Klok FA, Mos IC, Huisman MV (2008) Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 178:425–430
Becattini C, Vedovati MC, Agnelli G (2007) Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation 116:427–433
Jimenez D, Uresandi F, Otero R, Lobo JL, Monreal M, Marti D, Zamora J, Muriel A, Aujesky D, Yusen RD (2009) Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism: systematic review and metaanalysis. Chest 136:974–982
Lankeit M, Konstantinides S (2010) Thrombolysis for pulmonary embolism: past, present and future. Thromb Haemost 103:877–883
Binder L, Pieske B, Olschewski M, Geibel A, Klostermann B, Reiner C, Konstantinides S (2005) N-terminal pro-brain natriuretic peptide or troponin testing followed by echocardiography for risk stratification of acute pulmonary embolism. Circulation 112:1573–1579
Kucher N, Wallmann D, Carone A, Windecker S, Meier B, Hess OM (2003) Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur Heart J 24:1651–1656
Lankeit M, Kempf T, Dellas C, Cuny M, Tapken H, Peter T, Olschewski M, Konstantinides S, Wollert KC (2008) Growth differentiation factor-15 for prognostic assessment of patients with acute pulmonary embolism. Am J Respir Crit Care Med 177:1018–1025
Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, Roy PM, Fine MJ (2005) Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 172:1041–1046
Jimenez D, Aujesky D, Moores L, Gomez V, Lobo JL, Uresandi F, Otero R, Monreal M, Muriel A, Yusen RD (2010) Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 170:1383–1389
Agterof MJ, Schutgens RE, Moumli N, Eijkemans MJ, van der Griend R, Tromp EA, Biesma DH (2011) A prognostic model for short term adverse events in normotensive patients with pulmonary embolism. Am J Hematol 86:646–649
Uresandi F, Otero R, Cayuela A, Cabezudo MA, Jimenez D, Laserna E, Conget F, Oribe M, Nauffal D (2007) A clinical prediction rule for identifying short-term risk of adverse events in patients with pulmonary thromboembolism. Arch Bronconeumol 43:617–622
Wicki J, Perrier A, Perneger TV, Bounameaux H, Junod AF (2000) Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost 84:548–552
Lankeit M, Friesen D, Aschoff J, Dellas C, Hasenfuss G, Katus H, Konstantinides S, Giannitsis E (2010) Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur Heart J 31:1836–1844
Kurz K, Giannitsis E, Becker M, Hess G, Zdunek D, Katus HA (2011) Comparison of the new high sensitive cardiac troponin T with myoglobin, h-FABP and cTnT for early identification of myocardial necrosis in the acute coronary syndrome. Clin Res Cardiol 100:209–215
Kaczynska A, Pelsers MM, Bochowicz A, Kostrubiec M, Glatz JF, Pruszczyk P (2006) Plasma heart-type fatty acid binding protein is superior to troponin and myoglobin for rapid risk stratification in acute pulmonary embolism. Clin Chim Acta 371:117–123
Puls M, Dellas C, Lankeit M, Olschewski M, Binder L, Geibel A, Reiner C, Schafer K, Hasenfuss G, Konstantinides S (2007) Heart-type fatty acid-binding protein permits early risk stratification of pulmonary embolism. Eur Heart J 28:224–229
Dellas C, Puls M, Lankeit M, Schafer K, Cuny M, Berner M, Hasenfuss G, Konstantinides S (2010) Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol 55:2150–2157
Chan CP, Sum KW, Cheung KY, Glatz JF, Sanderson JE, Hempel A, Lehmann M, Renneberg I, Renneberg R (2003) Development of a quantitative lateral-flow assay for rapid detection of fatty acid-binding protein. J Immunol Methods 279:91–100
Alhashemi JA (2006) Diagnostic accuracy of a bedside qualitative immunochromatographic test for acute myocardial infarction. Am J Emerg Med 24:149–155
Mad P, Domanovits H, Fazelnia C, Stiassny K, Russmuller G, Cseh A, Sodeck G, Binder T, Christ G, Szekeres T, Laggner A, Herkner H (2007) Human heart-type fatty-acid-binding protein as a point-of-care test in the early diagnosis of acute myocardial infarction. QJM 100:203–210
Boscheri A, Wunderlich C, Langer M, Schoen S, Wiedemann B, Stolte D, Elmer G, Barthel P, Strasser RH (2010) Correlation of heart-type fatty acid-binding protein with mortality and echocardiographic data in patients with pulmonary embolism at intermediate risk. Am Heart J 160:294–300
(2000) Guidelines on diagnosis and management of acute pulmonary embolism. Task Force on Pulmonary Embolism, European Society of Cardiology. Eur Heart J 21:1301–1336
(2003) British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax 58:470–483
Konstantinides S (2008) Clinical practice. Acute pulmonary embolism. N Engl J Med 359:2804–2813
Sanchez O, Trinquart L, Colombet I, Durieux P, Huisman MV, Chatellier G, Meyer G (2008) Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 29:1569–1577
Colli A, Josa M, Pomar JL, Mestres CA, Gherli T (2007) Heart fatty acid binding protein in the diagnosis of myocardial infarction: where do we stand today? Cardiology 108:4–10
Scridon T, Scridon C, Skali H, Alvarez A, Goldhaber SZ, Solomon SD (2005) Prognostic significance of troponin elevation and right ventricular enlargement in acute pulmonary embolism. Am J Cardiol 96:303–305
ten Wolde M, Sohne M, Quak E, Mac Gillavry MR, Buller HR (2004) Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 164:1685–1689
Sanchez O, Trinquart L, Caille V, Couturaud F, Pacouret G, Meneveau N, Verschuren F, Roy PM, Parent F, Righini M, Perrier A, Lorut C, Tardy B, Benoit MO, Chatellier G, Meyer G (2010) Prognostic factors for pulmonary embolism: the prep study, a prospective multicenter cohort study. Am J Respir Crit Care Med 181:168–173
Acknowledgments
The study was supported by a grant from the University of Göttingen (Heidenreich von Siebold Programme) to C.D.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lankeit, M., Friesen, D., Schäfer, K. et al. A simple score for rapid risk assessment of non-high-risk pulmonary embolism. Clin Res Cardiol 102, 73–80 (2013). https://doi.org/10.1007/s00392-012-0498-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-012-0498-1