Abstract
Background
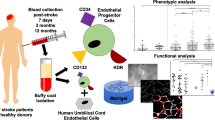
Buerger’s disease often shows poor collateral artery generation (i.e. neovascularization) in the ischemic limbs. However, the etiology has not yet been clarified. Circulating endothelial progenitor cells (EPCs) derived from bone marrow contribute to neovascularization in the multi-step process which includes the following capacities; mobilization, differentiation, adhesion, migration, invasion and secretion.
Materials and methods
We assessed EPCs capacities in vitro and ex vivo in age- and sex-matched controls (n = 12) and patients with Buerger’s disease (n = 12), derived from peripheral blood-derived mononuclear cells (PB-MNCs).
Results
In the flow cytometry analysis, the numbers of circulating EPC (CD34+/KDR+ or CD133+/KDR+ PB-MNC) were similar between controls and patients with Buerger’s disease. Next, we cultured PB-MNC to obtain EPCs. The number of early outgrowth EPCs was significantly decreased in patients with Buerger’s disease (p < 0.005), indicating the reduced generation of early outgrowth EPCs in Buerger’s disease. However, adhesion, migration, invasion and secretion capacities were not impaired in patients with Buerger’s disease.
Conclusions
The early outgrowth EPCs generation is reduced in patients with Buerger’s disease.

Similar content being viewed by others
Abbreviations
- EPCs:
-
Endothelial progenitor cells
- PB-MNCs:
-
Peripheral blood-derived mononuclear cells
- TAO:
-
Thromboangiitis obliterans
- ECs:
-
Endothelial cells
- KDR:
-
Kinase domain receptor
- VEGF:
-
Vascular endothelial growth factor
- FACS:
-
Fluorescence-activated cell sorting
- HUVEC:
-
Human umbilical vein endothelial cell
- Dil-acLDL:
-
1,1′-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein
- DAPI:
-
4′,6-diamidino-2-phenylindole
- b-FGF:
-
Basic fibroblast growth factor
- PDGF-BB:
-
Platelet-derived growth factor BB
- IGF:
-
Insulin growth factor
- SDF-1α:
-
Stromal cell-derived factor-1α
- ELISA:
-
Enzyme-linked immunosorbent assay
- IL-1, -6, -8:
-
Interleukin-1, -6, -8
- TGF-1α:
-
Tumor growth factor-1α
- VCAM-1:
-
Vascular cell adhesion molecule-1
- MCP-1:
-
Monocyte chemotactic protein-1
- hs-CRP:
-
High-sensitivity C-reactive protein
- m-RNA:
-
Messenger ribonucleic acid
References
Olin JW (2000) Thromboangiitis obliterans (Buerger’s disease). N Engl J Med 343:864–869
von Winiwarter F (1879) Ueber eine eigenthumliche Form von Endarteriitis und Endophlebitis mit Gangran des Fusses. Arch Klin Chir 23:202–206
Buerger L (1908) Thrombo-angiitis obliterans: a study of the vascular lesions leading to presenile spontaneous gangrene. Am J Med Sci 136:567–580
Folkmann J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31
Risau W (2000) Mechanisms of angiogenesis. Nature 386:671–674
Cameliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–395
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A et al (1998) Evidence for circulating bone marrow-derived endothelial cells. Blood 92:362–367
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M et al (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85:221–228
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95:343–353
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA et al (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348:593–600
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H et al (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89:E1–E7
Jung C, Fischer N, Fritzenwanger M, Thude H, Ferrari M, Fabris M et al (2009) Endothelial progenitor cells in adolescents: impact of overweight, age, smoking, sport and cytokines in younger age. Clin Res Cardiol 98:179–188
Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH et al (2004) Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 109:1615–1622
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A et al (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353:999–1007
Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U et al (2005) Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 111:2981–2987
Shionoya S (1998) Diagnostic criteria of Buerger’s disease. Int J Cardiol 66:S243–S245
Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M et al (2000) Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood 95:952–958
Hirschi KK, Ingram DA, Yoder MC (2008) Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28:1584–1595
Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR et al (2005) Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med 11:206–213
Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG et al (2001) Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci 938:36–45
Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M et al (2001) Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med 193:1005–1014
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM (2001) HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 108:391–397
Nishioka K, Higashi Y, Umemura T, Jituki D, Goto C, Nakamura S, et al. (2008) Vascular function and endothelial progenitor cells in thromboangiitis obliterans (Buerger’s disease). Circulation 118:S_635
Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Seidinger D et al (2005) Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation 111:204–211
Herbrig K, Haensel S, Oelschlaegel U, Pistrosch F, Foerster S, Passauer J (2006) Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann Rheum Dis 65:157–163
Moonen JR, de Leeuw K, van Seijen XJ, Kallenberg CG, van Luyn MJ, Bijl M et al (2007) Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Arthritis Res Ther 9:R84
Ebner P, Picard F, Richter J, Darrelmann E, Schneider M, Strauer BE et al (2010) Accumulation of VEGFR-2+/CD133+ cells and decreased number and impaired functionality of CD34+/VEGFR-2+ cells in patients with SLE. Rheumatology (Oxford) 49:63–72
Yamamoto K, Kondo T, Suzuki S, Izawa H, Kobayashi M, Emi N et al (2004) Molecular evaluation of endothelial progenitor cells in patients with ischemic limbs: therapeutic effect by stem cell transplantation. Arterioscler Thromb Vasc Biol 24:e192–e196
Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS et al (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380:439–442
Fong GH, Klingensmith J, Wood CR, Rossant J, Breitman ML (1996) Regulation of flt-1 expression during mouse embryogenesis suggests a role in the establishment of vascular endothelium. Dev Dyn 207:1–10
Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML et al (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376:62–66
Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH et al (2004) C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation 109:2058–2067
Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W et al (2006) Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab 28:90–98
Acknowledgments
We thank to Kimiko Kimura in the cardiovascular research institute, Kurume University for her excellent technical supports in the flow cytometric analysis and ELISA. This study was supported by Research Grants from The Ministry of Education, Science and Culture, Research on Human Genome, Tissue Engineering Food Biotechnology, and the Ministry of Health, Labor and Welfare, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, Kaibara Morikazu Medical Science Promotion Foundation and Kimura Memorial Heart Foundation Research, Japan.
Conflict of interest statement
There is no conflict of interest or financial disclosure by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katsuki, Y., Sasaki, Ki., Toyama, Y. et al. Early outgrowth EPCs generation is reduced in patients with Buerger’s disease. Clin Res Cardiol 100, 21–27 (2011). https://doi.org/10.1007/s00392-010-0198-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-010-0198-7