Abstract
Purpose
This study aimed to analyze the effect of ascitic carcinoembryonic antigen (CEA) levels on the long-term oncologic outcomes of colorectal cancer (CRC) following curative treatment.
Methods
A total of 191 patients with stage II/III CRC were included. CEA was analyzed on the peritoneal fluid samples taken at the start of each surgery. Long-term oncologic outcomes were analyzed using known risk factors for recurrence in CRC.
Result
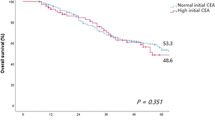
Multivariate analysis of recurrence showed that lymphatic invasion (hazards ratio (HR) 2.7, 95% confidence interval (CI) 1.1–7, p = 0.038), vascular invasion (HR 2.8, 95% CI 1.2–6.3, p = 0.013), mucinous cancer (HR 3.6, 95% CI 1.3–10.1, p = 0.017), and peritoneal fluid CEA exceeding 5 ng/dl (odds ratio 3.1, 95% CI 1.2–7.7, p = 0.017) were significant risk factors. There were 14 patients with liver metastasis, 11 of whom had high ascitic CEA levels and no peritoneal metastasis. Additionally, eight had lung metastasis, and seven of them had high ascitic CEA levels.
Conclusion
High ascitic CEA levels showed significantly lower disease-free survival and were significantly associated with distant metastasis in the lung and liver.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Cook AD, Single R, McCahill LE (2005) Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 12:637–645
Desch CE, Benson AB 3rd, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL et al (2005) Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 23:8512–8519
Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM (2010) Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut 59:1383–1388
Jeon CH, Kim MK, Lee IK (2019) Indication for and effect of adjuvant chemotherapy for stage IIa (T3N0M0) colon cancer. Ann Coloproctol 35:254–261
Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A (2012) Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 99:699–705
André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351
Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ et al (2011) Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 29:3768–3774
Hari DM, Leung AM, Lee JH, Sim MS, Vuong B, Chiu CG et al (2013) AJCC cancer staging manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment? J Am Coll Surg 217:181–90
Ambrose NS, MacDonald F, Young J, Thompson H, Keighley MR (1989) Monoclonal antibody and cytological detection of free malignant cells in the peritoneal cavity during resection of colorectal cancer—can monoclonal antibodies do better? Eur J Surg Oncol 15:99–102
Leather AJ, Kocjan G, Savage F, Hu W, Yiu CY, Boulos PB et al (1994) Detection of free malignant cells in the peritoneal cavity before and after resection of colorectal cancer. Dis Colon Rectum 37:814–819
Horattas MC, Evasovich MR, Topham N (1997) Colorectal carcinoma and the relationship of peritoneal cytology. Am J Surg 174:334–7. discussion 7–8
Schott A, Vogel I, Krueger U, Kalthoff H, Schreiber HW, Schmiegel W et al (1998) Isolated tumor cells are frequently detectable in the peritoneal cavity of gastric and colorectal cancer patients and serve as a new prognostic marker. Ann Surg 227:372–379
Kim BC, Bae JH, Park SM, Won DY, Lee IK (2020) Is ascites CEA a risk factor for peritoneal carcinomatosis in colorectal cancer?: a long-term follow-up study. Int J Colorectal Dis 35:147–155
Lee CS, Yoon SH, Lee SM, Lee IK, Kim JY, Cho HM et al (2020) Micropuncture access set use during implantation of totally implantable venous access device may reduce upper extremity DVT incidence among patients undergoing chemotherapy for colorectal cancer. World J Surg 44:1302–1308
Glimelius B, Cavalli-Björkman N (2012) Metastatic colorectal cancer: current treatment and future options for improved survival. Medical approach—present status. Scand J Gastroenterol 47:296–314
Yamamoto M, Baba H, Kakeji Y, Endo K, Ikeda Y, Toh Y et al (2004) Prognostic significance of tumor markers in peritoneal lavage in advanced gastric cancer. Oncology 67:19–26
Lee IK, Kim DH, Gorden DL, Lee YS, Sung NY, Park GS et al (2009) Prognostic value of CEA and CA 19–9 tumor markers combined with cytology from peritoneal fluid in colorectal cancer. Ann Surg Oncol 16:861–870
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331:1559–1564
Cao H, Xu E, Liu H, Wan L, Lai M (2015) Epithelial-mesenchymal transition in colorectal cancer metastasis: a system review. Pathol Res Pract 211:557–569
Kanda M, Kodera Y (2016) Molecular mechanisms of peritoneal dissemination in gastric cancer. World J Gastroenterol 22:6829–6840
Kasagi Y, Harada Y, Morodomi Y, Iwai T, Saito S, Yoshida K et al (2016) Peritoneal dissemination requires an Sp1-dependent CXCR4/CXCL12 signaling axis and extracellular matrix-directed spheroid formation. Cancer Res 76:347–357
Masiello T, Dhall A, Hemachandra LPM, Tokranova N, Melendez JA, Castracane J (2018) A dynamic culture method to produce ovarian cancer spheroids under physiologically-relevant shear stress. Cells 7
Shield K, Ackland ML, Ahmed N, Rice GE (2009) Multicellular spheroids in ovarian cancer metastases: biology and pathology. Gynecol Oncol 113:143–148
Sodek KL, Ringuette MJ, Brown TJ (2009) Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int J Cancer 124:2060–2070
Lengyel E (2010) Ovarian cancer development and metastasis. Am J Pathol 177:1053–1064
Terao K, Uchiumi T, Ogata K (1980) Cross-linking of L5 protein to 5 S RNA in rat liver 60-S subunits by ultraviolet irradiation. Biochim Biophys Acta 609:306–312
Riihimäki M, Hemminki A, Sundquist J, Hemminki K (2016) Patterns of metastasis in colon and rectal cancer. Sci Rep 6:29765
Author information
Authors and Affiliations
Contributions
Conceptualization and design: I.K.L. and C.S.L.; patient data and samples: A.A.S., H.J.L., and J.H.B.; experiments, collection, and assembly of data: Y.S.L., M.R.Y., and S.R.H.; data analysis and interpretation: C.S.L. and J.H.B.; manuscript writing and editing: A.A.S., C.S.L., and I.K.L.; critical revision: D.L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by St. Mary’s Hospital Research Ethics Board (KC17TESI0796) and waived the requirement for informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Sawat, A., Bea, J.H., Han, SR. et al. Use of ascitic CEA levels as a predictive value for distant metastasis in high-risk stage II and III colorectal cancer. Int J Colorectal Dis 37, 365–372 (2022). https://doi.org/10.1007/s00384-021-04070-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-021-04070-x