Abstract
Background
Anal cancer is a rare entity and the effect of gender and HPV status on survival is controversial. We aimed to evaluate the difference in overall survival (OS) according to gender and analyzed the effect of HPV status on OS.
Patients and methods
The National Cancer Database (NCDB) was queried for patients with anal squamous cell carcinoma between 2004 and 2016. We evaluated the OS based on gender and HPV status using Kaplan-Meier estimates and we used multivariate Cox regression analyses to evaluate factors associated with overall survival.
Results
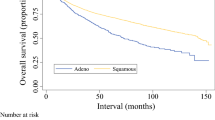
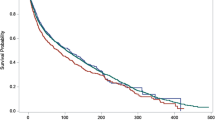
A total of 6133 patients with known HPV status were included for analysis. In the non-metastatic group, male gender was associated with worse OS (HR 1.50, 95% CI 1.32–1.70; P<0.001) whereas HPV status did not affect the OS (HR 1.08, 95% CI 0.96–1.22; P=0.213). In the metastatic group, there was no difference in OS based on gender (HR 1.29, 95% CI 0.91–1.82; P=0.148), whereas HPV-negative status was associated with worse OS (HR 1.52, 95% CI 1.09–2.12; P=0.014).
Conclusion
Females had better OS only in non-metastatic anal squamous cell carcinoma (ASCC). HPV-negative status was associated with worse OS only in metastatic ASCC.




Similar content being viewed by others
Data availability
The data we used in this study is available from the National Cancer Database.
References
Fields AC, Welten VM, Lu P et al (2019) Does race impact survival for patients with anal squamous cell carcinoma? J Surg Oncol 120:1201–1207
American Cancer Society. Key statistics for anal cancer. 2020. Available from: https://www.cancer.org/cancer/anal-cancer/about/what-is-key-statistics.html. Accessed 10 Nov 2020
Valvo F, Ciurlia E, Avuzzi B et al (2019) Cancer of the anal region. Crit Rev Oncol Hematol 135:115–127. https://doi.org/10.1016/j.critrevonc.2018.12.007
Baidoun F, Saad AM, Gad M et al (2020) Gender disparities in anal cancer incidence and mortality: A long-term US population-based analysis. J Clin Oncol 38:e16059 Available from: https://meetinglibrary.asco.org/record/188872/abstract. Accessed 10 Nov 2020
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34
National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) program. Available from: https://seer.cancer.gov/statfacts/html/anus.html. Accessed 10 Nov 2020
Nelson VM, Benson AB (2017) Epidemiology of anal canal cancer. Surg Oncol Clin N Am 26:9–15. https://doi.org/10.1016/j.soc.2016.07.001
Sun G, Dong X, Tang X et al (2018) The prognostic value of HPV combined p16 status in patients with anal squamous cell carcinoma: a meta-analysis. Oncotarget. 9:8081–8088
Morris VK, Rashid A, Rodriguez-Bigas M et al (2015) Clinicopathologic features associated with human papillomavirus/p16 in patients with metastatic squamous cell carcinoma of the anal canal. Oncologist. 20:1247–1252
Balsitis SJ, Sage J, Duensing S et al (2003) Recapitulation of the effects of the human papillomavirus type 16 E7 oncogene on mouse epithelium by somatic Rb deletion and detection of pRb-independent effects of E7 in vivo. Mol Cell Biol 23:9094–9103 Available from: https://mcb.asm.org/content/23/24/9094. Accessed 15 Nov 2020
Ruiz S (2004) Unique and overlapping functions of pRb and p107 in the control of proliferation and differentiation in epidermis. Development 131:2737–2748 Available from: http://dev.biologists.org/cgi/doi/10.1242/dev.01148. Accessed 15 Nov 2020
Daling JR, Madeleine MM, Johnson LG et al (2004) Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 101:270–280
Ang KK, Harris J, Wheeler R et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35 Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa0912217. Accessed 15 Nov 2020
Vosmik M, Laco J, Sirak I et al (2014) Prognostic significance of human papillomavirus (HPV) status and expression of selected markers (HER2/neu, EGFR, VEGF, CD34, p63, p53 and Ki67/MIB-1) on outcome after (chemo-) radiotherapy in patients with squamous cell carcinoma of uterine cervix. Pathol Oncol Res 20:131–137 Available from: http://link.springer.com/10.1007/s12253-013-9674-5. Accessed 15 Nov 2020
Wan Z, Huang Z, Vikash V et al (2017) Survival rate variation with different histological subtypes of poor prognostic male anal squamous cell carcinoma: a population-based study. Oncotarget 8:84349–84359 Available from: https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.20969. Accessed 15 Nov 2020
Gupta A, Arora N, Zhu H et al (2017) Racial and gender disparities in therapy and outcomes of squamous cell cancer of the anus. J Clin Oncol 35:691 Available from: http://ascopubs.org/doi/10.1200/JCO.2017.35.4_suppl.691. Accessed 15 Nov 2020
Celie KB, Jackson C, Agrawal S et al (2017) Socioeconomic and gender disparities in anal cancer diagnosis and treatment. Surg Oncol 26:212–217. https://doi.org/10.1016/j.suronc.2017.03.008
Razzaghi H, Saraiya M, Thompson TD et al (2018)Five-year relative survival for human papillomavirus-associated cancer sites. Cancer 124:203–211 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0031938416312148
National Cancer Database - About the National Cancer Database. Available from: https://www.facs.org/quality-programs/cancer/ncdb/about. Accessed on Septemeber 8th, 2020
Ravenda PS (2015) Human papillomavirus in anal squamous cell carcinoma: An angel rather than a devil? Ecancermedicalscience 9:2–5 Available from: http://www.ecancer.org/journal/9/full/529-human-papillomavirus-in-anal-squamous-cell-carcinoma-an-angel-rather-than-a-devil.php. Accessed 18 Nov 2020
Yao JN, Zhang XX, Zhou HN et al (2017) Human papillomavirus related anal squamous cell carcinoma survival: A systematic review and meta-analysis. Transl Cancer Res 6:463–473
Jhaveri J, Rayfield L, Liu Y et al (2017) Prognostic relevance of human papillomavirus infection in anal squamous cell carcinoma: Analysis of the National Cancer Database. J Gastrointest Oncol 8:998–1008
Kimple RJ, Smith MA, Blitzer GC et al (2013) Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res 73:4791–4800 Available from: http://cancerres.aacrjournals.org/cgi/doi/10.1158/0008-5472.CAN-13-0587. Accessed 18 Nov 2020
Rieckmann T, Tribius S, Grob TJ et al (2013) HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol 107:242 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167814013001370. Accessed 18 Nov 2020
Yhim HY, Lee NR, Song EK et al (2011) The prognostic significance of tumor human papillomavirus status for patients with anal squamous cell carcinoma treated with combined chemoradiotherapy. Int J Cancer 129:1752–1760
Westra WH, Taube JM, Poeta ML et al (2008) Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res 14:366–369 Available from: http://clincancerres.aacrjournals.org/cgi/doi/10.1158/1078-0432.CCR-07-1402. Accessed 18 Nov 2020
Meulendijks D, Tomasoa NB, Dewit L et al (2015)HPV-negative squamous cell carcinoma of the anal canal is unresponsive to standard treatment and frequently carries disruptive mutations in TP53. Br J Cancer 112:1358–1366. https://doi.org/10.1038/bjc.2015.20
Ravenda PS, Magni E, Botteri E et al (2014) Prognostic value of human papillomavirus in anal squamous cell carcinoma. Cancer Chemother Pharmacol 74:1033–1038 Available from: http://link.springer.com/10.1007/s00280-014-2582-x. Accessed 18 Nov 2020
Laytragoon-Lewin N, Nilsson PJ, Castro J et al (2007) Human papillomavirus (HPV), DNA aberrations and cell cycle progression in anal squamous cell carcinoma patients. Anticancer Res 27:4473–4479
McLaughlin-Drubin ME, Park D, Munger K (2013) Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc Natl Acad Sci 110:16175–16180 Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.1310432110. Accessed 18 Nov 2020
Lassen P, Eriksen JG, Hamilton-Dutoit S et al (2009) Effect of HPV-associated p16 INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 27:1992–1998 Available from: http://ascopubs.org/doi/10.1200/JCO.2008.20.2853. Accessed 18 Nov 2020
Gilbert DC, Williams A, Allan K et al (2013) p16INK4A, p53, EGFR expression and KRAS mutation status in squamous cell cancers of the anus: Correlation with outcomes following chemo-radiotherapy. Radiother Oncol 109:146–151 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167814013003745. Accessed 18 Nov 2020
Hoffmann M, Ihloff AS, Görögh T et al (2010) p16INK4a overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer 127:1595–1602. https://doi.org/10.1002/ijc.25174
Baricevic I, He X, Chakrabarty B et al (2015)High-sensitivity human papilloma virus genotyping reveals near universal positivity in anal squamous cell carcinoma: different implications for vaccine prevention and prognosis. Eur J Cancer 51:776–785. https://doi.org/10.1016/j.ejca.2015.01.058
Serup-Hansen E, Linnemann D, Skovrider-Ruminski W et al (2014) Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol 32:1812–1817
Mai S, Welzel G, Ottstadt M et al (2015) Prognostic relevance of HPV infection and p16 overexpression in squamous cell anal cancer. Int J Radiat Oncol Biol Phys 93:819–827. https://doi.org/10.1016/j.ijrobp.2015.08.004
Ahmad TR, Susko M, Lindquist K et al (2019) Socioeconomic disparities in timeliness of care and outcomes for anal cancer patients. Cancer Med 8:7186–7196
Ramey SJ, Rich BJ, Kwon D et al (2018) Demographic disparities in delay of definitive chemoradiation for anal squamous cell carcinoma: A nationwide analysis. J Gastrointest Oncol 9:1109–1126
Lin D, Gold HT, Schreiber D et al (2018) Impact of socioeconomic status on survival for patients with anal cancer. Cancer. 124:1791–1797
Ajani JA, Winter KA, Gunderson LL et al (2010) Prognostic factors derived from a prospective database dictate clinical biology of anal cancer: The intergroup trial (RTOG 98-11). Cancer. 116:4007–4013
Glynne-Jones R, Sebag-Montefiore D, Adams R et al (2013) Prognostic factors for recurrence and survival in anal cancer: Generating hypotheses from the mature outcomes of the first United Kingdom Coordinating Committee on Cancer Research Anal Cancer Trial (ACT I). Cancer. 119:748–755
Silva Dalla Libera L, Almeida De Carvalho KP, Enocencio Porto Ramos J et al (2019) Human papillomavirus and anal cancer: Prevalence, genotype distribution, and prognosis aspects from midwestern region of Brazil. J Oncol 2019
Author information
Authors and Affiliations
Contributions
Firas Baidoun: data analysis, data interpretation, manuscript writing.
Anas M. Saad: manuscript editing, critical revision.
Omar Abdel-Rahman: study concept, manuscript editing, critical revision.
Corresponding author
Ethics declarations
Consent
Not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Article highlights
• Anal cancer is a rare malignancy and anal squamous cell carcinoma (ASCC) accounts for about 80% of it.
• Human papillomavirus (HPV) is the most common risk factor associated with ASCC.
• The prognostic effect of HPV status in ASCC is still controversial and not well-established.
• There are increasing data about gender disparity in ASCC prognosis, but still conflicting and controversial.
• We found that females had better OS only in non-metastatic ASCC, whereas HPV-negative status was associated with worse OS only in metastatic ASCC.
Rights and permissions
About this article
Cite this article
Baidoun, F., Saad, A.M. & Abdel-Rahman, O. The impact of gender and HPV status on anal squamous cell carcinoma survival. Int J Colorectal Dis 36, 2093–2109 (2021). https://doi.org/10.1007/s00384-021-03910-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-021-03910-0