Abstract
Purpose
Angiostatin and angiostatin-like molecules are known as anti-angiogenic factors, which inhibit endothelial cell functions resulting in reduced tumour growth. Recent data indicate that these molecules, especially PlgK1-5, directly affect tumour cells, which could explain the strong anti-tumoural effects of PlgK1-5. Therefore, we have analysed whether PlgK1-5 alters tumour cell functions and expression levels of cell adhesion molecules in murine and human hepatoma cells in vitro and in vivo.
Methods
First, effects on tumour growth, proliferation and apoptosis were investigated in vivo in a subcutaneous tumour model. In vitro, effects of PlgK1-5 on tumour cell apoptosis, clonal expansion, migration, corresponding ICAM expression and intracellular signal transduction in murine Hepa129 and human HuH7 hepatoma cells have been analysed.
Results
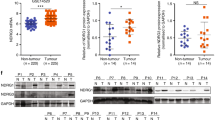
In vivo, subcutaneous tumour growth was reduced by 75% in PlgK1-5-treated animals compared to the controls. This was accompanied by increased tumour cell apoptosis (up to 33%) and decreased tumour cell proliferation (by up to 21%). In vitro, PlgK1-5 induced apoptosis in hepatoma cells, corresponding to increased caspase-8 cleavage and reduced AKT phosphorylation. Migration and clonal expansion was also diminished in PlgK1-5-treated Hepa129, corresponding to decreased ICAM expression levels.
Conclusions
Here, we show that PlgK1-5 directly affects tumour cells by decreasing cell adhesion resulting—at least partly—in apoptosis. This is mediated by altered intracellular signal transduction and by activation of the caspase cascade. These findings further underscore the potential therapeutic role of PlgK1-5 in the treatment of HCC.








Similar content being viewed by others
References
Llovet J, Ricci S, Mazzaferro V, Hilgard P, Raoul J, Zeuzem S (2007) Sorafenib improves survival in advanced hepatocellular carcinoma (HCC): results of a phase III randomized placebo-controlled trial (SHARP trial). J Clin Oncol 25 (Suppl.):18
Hanford HA, Wong CA, Kassan H, Cundiff DL, Chandel N, Underwood S, Mitchell CA, Soff GA (2003) Angiostatin(4.5)-mediated apoptosis of vascular endothelial cells. Cancer Res 63(14):4275–4280
Schmitz V, Wang L, Barajas M, Peng D, Prieto J, Qian C (2002) A novel strategy for the generation of angiostatic kringle regions from a precursor derived from plasminogen. Gene Ther 9(23):1600–1606
Schmitz V, Raskopf E, Gonzalez-Carmona MA, Vogt A, Rabe C, Leifeld L, Kornek M, Sauerbruch T, Caselmann WH (2007) Plasminogen fragment K1-5 improves survival in a murine hepatocellular carcinoma model. Gut 56(2):271–278
Schmitz V, Raskopf E, Gonzalez-Carmona MA, Vogt A, Kornek M, Sauerbruch T, Caselmann WH (2008) Plasminogen derivatives encoding kringles 1–4 and kringles 1–5 of exert indirect antiangiogenic and direct antitumoral effects in experimental lung cancer. Cancer Invest 26(5):464–470
Chen YH, Wu HL, Li C, Huang YH, Chiang CW, Wu MP, Wu LW (2006) Anti-angiogenesis mediated by angiostatin K1-3, K1-4 and K1-4.5. Involvement of p53, FasL, AKT and mRNA deregulation. Thromb Haemost 95(4):668–677
Ji WR, Castellino FJ, Chang Y, Deford ME, Gray H, Villarreal X, Kondri ME, Marti DN, Llinas M, Schaller J, Kramer RA, Trail PA (1998) Characterization of kringle domains of angiostatin as antagonists of endothelial cell migration, an important process in angiogenesis. FASEB J 12(15):1731–1738
Eriksson K, Magnusson P, Dixelius J, Claesson-Welsh L, Cross MJ (2003) Angiostatin and endostatin inhibit endothelial cell migration in response to FGF and VEGF without interfering with specific intracellular signal transduction pathways. FEBS Lett 536(1–3):19–24
Schmitz V, Wang L, Barajas M, Gomar C, Prieto J, Qian C (2004) Treatment of colorectal and hepatocellular carcinomas by adenoviral mediated gene transfer of endostatin and angiostatin-like molecule in mice. Gut 53(4):561–567
Perri SR, Nalbantoglu J, Annabi B, Koty Z, Lejeune L, Francois M, Di Falco MR, Beliveau R, Galipeau J (2005) Plasminogen kringle 5-engineered glioma cells block migration of tumor-associated macrophages and suppress tumor vascularization and progression. Cancer Res 65(18):8359–8365
Chen YH, Wu HL, Chen CK, Huang YH, Yang BC, Wu LW (2003) Angiostatin antagonizes the action of VEGF-A in human endothelial cells via two distinct pathways. Biochem Biophys Res Commun 310(3):804–810
Lee TY, Muschal S, Pravda EA, Folkman J, Abdollahi A, Javaherian K (2009) Angiostatin regulates the expression of antiangiogenic and proapoptotic pathways via targeted inhibition of mitochondrial proteins. Blood 114(9):1987–1998. doi:10.1182/blood-2008-12-197236
Chi SL, Pizzo SV (2006) Angiostatin is directly cytotoxic to tumor cells at low extracellular pH: a mechanism dependent on cell surface-associated ATP synthase. Cancer Res 66(2):875–882
Veitonmaki N, Cao R, Wu LH, Moser TL, Li B, Pizzo SV, Zhivotovsky B, Cao Y (2004) Endothelial cell surface ATP synthase-triggered caspase-apoptotic pathway is essential for k1-5-induced antiangiogenesis. Cancer Res 64(10):3679–3686
Sharma MR, Rothman V, Tuszynski GP, Sharma MC (2006) Antibody-directed targeting of angiostatin's receptor annexin II inhibits Lewis Lung Carcinoma tumor growth via blocking of plasminogen activation: possible biochemical mechanism of angiostatin's action. Exp Mol Pathol 81(2):136–145
Raskopf E, Gerceker S, Vogt A, Standop J, Sauerbruch T, Schmitz V (2009) Plasminogen fragment K1-3 inhibits expression of adhesion molecules and experimental HCC recurrence in the liver. Int J Colorectal Dis 24(7):837–844. doi:10.1007/s00384-009-0652-z
Kornek M, Raskopf E, Guetgemann I, Ocker M, Gerceker S, Gonzalez-Carmona MA, Rabe C, Sauerbruch T, Schmitz V (2006) Combination of systemic thioacetamide (TAA) injections and ethanol feeding accelerates hepatic fibrosis in C3H/He mice and is associated with intrahepatic up regulation of MMP-2, VEGF and ICAM-1. J Hepatol 45(3):370–376
Cao Y, Chen A, An SS, Ji RW, Davidson D, Llinas M (1997) Kringle 5 of plasminogen is a novel inhibitor of endothelial cell growth. J Biol Chem 272(36):22924–22928
Cao Y, O'Reilly MS, Marshall B, Flynn E, Ji RW, Folkman J (1998) Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases. J Clin Invest 101(5):1055–1063. doi:10.1172/JCI1558
Gyorffy S, Palmer K, Gauldie J (2001) Adenoviral vector expressing murine angiostatin inhibits a model of breast cancer metastatic growth in the lungs of mice. Am J Pathol 159(3):1137–1147
Wahl ML, Owen CS, Grant DS (2002) Angiostatin induces intracellular acidosis and anoikis in endothelial cells at a tumor-like low pH. Endothelium 9(3):205–216
O'Reilly MS, Holmgren L, Chen C, Folkman J (1996) Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 2(6):689–692
Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW (2001) Angiostatin enhances B7.1-mediated cancer immunotherapy independently of effects on vascular endothelial growth factor expression. Cancer Gene Ther 8(10):719–727
Galaup A, Magnon C, Rouffiac V, Opolon P, Opolon D, Lassau N, Tursz T, Perricaudet M, Griscelli F (2005) Full kringles of plasminogen (aa 1–566) mediate complete regression of human MDA-MB-231 breast tumor xenografted in nude mice. Gene Ther 12(10):831–842
Kim YG, Kim MJ, Lim JS, Lee MS, Kim JS, Yoo YD (2006) ICAM-3-induced cancer cell proliferation through the PI3K/Akt pathway. Cancer Lett 239(1):103–110. doi:10.1016/j.canlet.2005.07.023
Park JK, Park SH, So K, Bae IH, Yoo YD, Um HD (2010) ICAM-3 enhances the migratory and invasive potential of human non-small cell lung cancer cells by inducing MMP-2 and MMP-9 via Akt and CREB. Int J Oncol 36(1):181–192
Acknowledgements
This work was supported by a grant from the H.W. and J. Hector-Stiftung and a Deutsche Krebshilfe grant to ER and VS. We thank Oliver Feuser and Ute Henseler for the proof reading of the manuscript.
Conflict of interest
No conflict of interests exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmitz, V., Sauerbruch, T. & Raskopf, E. Anti-tumoural effects of PlgK1-5 are directly linked to reduced ICAM expression, resulting in hepatoma cell apoptosis. Int J Colorectal Dis 27, 1029–1038 (2012). https://doi.org/10.1007/s00384-012-1418-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-012-1418-6