Abstract
Objectives
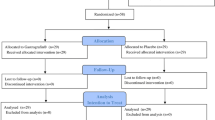
This was a prospective, randomised, placebo-controlled, double-blind multicentre trial to analyse the efficacy of choline citrate in patients with postoperative ileus (POI) after elective colorectal surgery.
Methods
From October 2005 until June 2008, 122 patients with POI were randomised to receive choline citrate or placebo. One hundred twenty patients were evaluable for tolerability and 107 patients were evaluable for efficacy. The treatment group, 47% (50/107), received 300.2 mg choline citrate intravenously, while the placebo group, 53% (57/107), received sodium chloride. Injections were performed every 12 h until defecation.
Results
Demographic data analysis did not show clinically differences between both groups. Operative procedures included 40% (43/107) hemicolectomy, 38% (41/107) sigmoid resection and 22% (23/107) other colorectal resections. Defecation occurred after an average of 91.8 ± 26.6 h postoperatively in the treatment group, vs. 96.7 ± 35.2 h in the placebo group (p = 0.805). After laparoscopy, defecation occurred after 78.7 ± 25.3 h, vs. 99.2 ± 31.6 h after laparotomy (p = 0.001). Serious adverse effects occurred in 2% (1/60) in the treatment group, vs. 3% (2/60) in the placebo group. None of the events have been assessed as related to the study medication.
Conclusion
An efficacy of choline citrate in the treatment of POI after elective colorectal surgery could not be verified. The problem of POI requiring drug treatment seems to be less frequent than suggested by the literature. With technical advances in surgery, especially laparoscopic and fast track surgery, the frequency of POI will further decrease in the future.


Similar content being viewed by others
References
Holte K, Kehlet H (2000) Postoperative ileus: a preventable event. Br J Surg 87(11):1480–1493
Bauer AJ, Boeckxstaens GE (2004) Mechanisms of postoperative ileus. Neurogastroenterol Motil 16(Suppl 2):54–60
Senagore AJ (2007) Pathogenesis and clinical and economic consequences of postoperative ileus. Am J Health Syst Pharm 64(20 Suppl 13):S3–S7
Luckey A, Livingston E, Tache Y (2003) Mechanisms and treatment of postoperative ileus. Arch Surg 138(2):206–214
Livingston EH, Passaro EP Jr (1990) Postoperative ileus. Dig Dis Sci 35(1):121–132
Waldhausen JH, Shaffrey ME, Skenderis BS 2nd et al (1990) Gastrointestinal myoelectric and clinical patterns of recovery after laparotomy. Ann Surg 211(6):777–784, discussion 785
Iyer S, Saunders WB, Stemkowski S (2009) Economic burden of postoperative ileus associated with colectomy in the United States. J Manag Care Pharm 15(6):485–494
Smith AJ, Nissan A, Lanouette NM et al (2000) Prokinetic effect of erythromycin after colorectal surgery: randomized, placebo-controlled, double-blind study. Dis Colon Rectum 43(3):333–337
Cheape JD, Wexner SD, James K, Jagelman DG (1991) Does metoclopramide reduce the length of ileus after colorectal surgery? A prospective randomized trial. Dis Colon Rectum 34(6):437–441
Brown TA, McDonald J, Williard W (1999) A prospective, randomized, double-blinded, placebo-controlled trial of cisapride after colorectal surgery. Am J Surg 177(5):399–401
Holte K, Kehlet H (2002) Postoperative ileus: progress towards effective management. Drugs 62(18):2603–2615
Ballet F, Lecomte D, Petit J et al (1982) Idiopathic intestinal pseudo-obstruction. Apropos of a case treated with cholinergic and adrenolytic agents. Gastroentérol Clin Biol 6(6–7):598
Petrila T, Balan A, Tulbure D (1981) A mixture of an alpha blocking agent and choline agonist in the treatment of postoperative intestinal paralysis. Rev Chir Oncol Radiol O R L Oftalmol Stomatol Chir 30(4):317–320
Holte K, Sharrock NE, Kehlet H (2002) Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth 89(4):622–632
Holte K, Foss NB, Andersen J et al (2007) Liberal or restrictive fluid administration in fast-track colonic surgery: a randomized, double-blind study. Br J Anaesth 99(4):500–508
Stewart BT, Woods RJ, Collopy BT et al (1998) Early feeding after elective open colorectal resections: a prospective randomized trial. Aust N Z J Surg 68(2):125–128
Jorgensen H, Wetterslev J, Moiniche S, Dahl JB (2000) Epidural local anaesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev 4:CD001893
Kehlet H, Wilmore DW (2002) Multimodal strategies to improve surgical outcome. Am J Surg 183(6):630–641
Kehlet H (2008) Postoperative ileus—an update on preventive techniques. Nat Clin Pract Gastroenterol Hepatol 5(10):552–558
Zmora O, Hashavia E, Munz Y et al (2009) Laparoscopic colectomy is associated with decreased postoperative gastrointestinal dysfunction. Surg Endosc 23(1):87–89
Roberts DJ, Banh HL, Hall RI (2006) Use of novel prokinetic agents to facilitate return of gastrointestinal motility in adult critically ill patients. Curr Opin Crit Care 12(4):295–302
Beaussier M, Weickmans H, Parc Y et al (2006) Postoperative analgesia and recovery course after major colorectal surgery in elderly patients: a randomized comparison between intrathecal morphine and intravenous PCA morphine. Reg Anesth Pain Med 31(6):531–538
Artinyan A, Nunoo-Mensah JW, Balasubramaniam S et al (2008) Prolonged postoperative ileus-definition, risk factors, and predictors after surgery. World J Surg 32(7):1495–1500
MacKay G, Ihedioha U, McConnachie A et al (2007) Laparoscopic colonic resection in fast-track patients does not enhance short-term recovery after elective surgery. Colorectal Dis 9(4):368–372
Teeuwen PH, Bleichrodt RP, Strik C et al (2009) Enhanced recovery after surgery (ERAS) versus conventional postoperative care in colorectal surgery. J Gastrointest Surg 25:25
Leung AM, Gibbons RL, Vu HN (2009) Predictors of length of stay following colorectal resection for neoplasms in 183 Veterans Affairs patients. World J Surg 33(10):2183–2188
Delaney CP, Wolff BG, Viscusi ER et al (2007) Alvimopan, for postoperative ileus following bowel resection: a pooled analysis of phase III studies. Ann Surg 245(3):355–363
Acknowledgments
The study was supported by the manufacturer of choline citrate (Neurotropan®), Phoenix Laboratorium, Germany, www.phoenix-lab.de.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript type: prospective, randomised, placebo-controlled, double-blind phase III trial
Trial registration study number: CC 0401, protocol version 6.0, July 7th, 2006
EUCRACT number: 2005-000182-19
Rights and permissions
About this article
Cite this article
Herzog, T., Lemmens, H.P., Arlt, G. et al. Treatment of postoperative ileus with choline citrate—results of a prospective, randomised, placebo-controlled, double-blind multicentre trial. Int J Colorectal Dis 26, 645–652 (2011). https://doi.org/10.1007/s00384-010-1092-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-010-1092-5