Abstract
Purpose
The majority of relapsed neuroblastomas have mitogen-activated protein kinase (MAPK) pathway activating mutations. We previously showed the in vitro and in vivo anti-tumor effects of MAPK/ERK kinase (MEK) inhibitors in MAPK-activated neuroblastoma. We herein assessed the correlation between MAPK activation and the prognosis in neuroblastoma patients using phosphorylated extra-cellular signal-regulated kinase (pERK) immunohistochemistry to establish the protocol for the clinical administration of MEK inhibitors.
Methods
Neuroblastoma samples from patients treated in our hospital were immunostained with pERK. The clinical outcomes were retrospectively collected from medical records. The correlation between pERK positivity and the prognosis was analyzed.
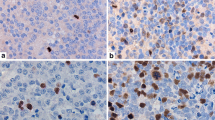
Results
Regarding pre-chemotherapeutic specimens, there were no differences in the pERK status between tumors with a good and bad prognosis in both the nuclei and cytoplasm. Regarding post-chemotherapeutic specimens, one of eight tumors with a good prognosis and four of six tumors with a poor prognosis showed pERK-positive nuclear staining (p = 0.0909) and five of eight tumors with a good prognosis and four of six tumors with a poor prognosis showed pERK-positive cytoplasmic staining (p > 0.9999).
Conclusion
These findings suggested post-chemotherapeutic—not pre-chemotherapeutic—nuclear pERK-positive neuroblastoma tends to be associated with a poor prognosis and may be a potential therapeutic target for MEK inhibitor treatment.




Similar content being viewed by others
References
Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, Weiss WA (2016) Neuroblastoma Nat Rev Dis Primers 2:16078. https://doi.org/10.1038/nrdp.2016.78
Greengard EG (2018) Molecularly targeted therapy for neuroblastoma. Children (Basel). https://doi.org/10.3390/children5100142
Cañete A (2020) High-risk neuroblastoma: where do we go? Ann Oncol 31(3):326–327. https://doi.org/10.1016/j.annonc.2019.12.003
Eleveld TF, Oldridge DA, Bernard V, Koster J, Daage LC, Diskin SJ, Schild L, Bentahar NB, Bellini A, Chicard M, Lapouble E, Combaret V, Legoix-Ne P, Michon J, Pugh TJ, Hart LS, Rader J, Attiyeh EF, Wei JS, Zhang S, Naranjo A, Gastier-Foster JM, Hogarty MD, Asgharzadeh S, Smith MA, Guidry Auvil JM, Watkins TB, Zwijnenburg DA, Ebus ME, van Sluis P, Hakkert A, van Wezel E, van der Schoot CE, Westerhout EM, Schulte JH, Tytgat GA, Dolman ME, Janoueix-Lerosey I, Gerhard DS, Caron HN, Delattre O, Khan J, Versteeg R, Schleiermacher G, Molenaar JJ, Maris JM (2015) Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet 47(8):864–871. https://doi.org/10.1038/ng.3333
Tanaka T, Higashi M, Kimura K, Wakao J, Fumino S, Iehara T, Hosoi H, Sakai T, Tajiri T (2016) MEK inhibitors as a novel therapy for neuroblastoma: their in vitro effects and predicting their efficacy. J Pediatr Surg 51(12):2074–2079. https://doi.org/10.1016/j.jpedsurg.2016.09.043
Takeuchi Y, Tanaka T, Higashi M, Fumino S, Iehara T, Hosoi H, Sakai T, Tajiri T (2018) In vivo effects of short- and long-term MAPK pathway inhibition against neuroblastoma. J Pediatr Surg 53(12):2454–2459. https://doi.org/10.1016/j.jpedsurg.2018.08.026
Wang H, Zhang Y, Yun H, Chen S, Chen Y, Liu Z (2017) ERK expression and its correlation with STAT1 in esophageal squamous cell carcinoma. Oncotarget 8(28):45249–45258. https://doi.org/10.18632/oncotarget.16902
Mawrin C, Diete S, Treuheit T, Kropf S, Vorwerk CK, Boltze C, Kirches E, Firsching R, Dietzmann K (2003) Prognostic relevance of MAPK expression in glioblastoma multiforme. Int J Oncol 23(3):641–648
Pelloski CE, Lin E, Zhang L, Yung WKA, Colman H, Liu J-L, Woo SY, Heimberger AB, Suki D, Prados M, Chang S, Barker FG, Fuller GN, Aldape KD (2006) Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in Glioblastoma. Clin Cancer Res 12(13):3935–3941. https://doi.org/10.1158/1078-0432.Ccr-05-2202
Holck S, Nielsen HJ, Pedersen N, Larsson L-I (2015) Phospho-ERK1/2 levels in cancer cell nuclei predict responsiveness to radiochemotherapy of rectal adenocarcinoma. Oncotarget 6(33):34321–34328. https://doi.org/10.18632/oncotarget.5761
Smith ER, Cai KQ, Smedberg JL, Ribeiro MM, Rula ME, Slater C, Godwin AK, Xu XX (2010) Nuclear entry of activated MAPK is restricted in primary ovarian and mammary epithelial cells. PLoS ONE 5(2):e9295. https://doi.org/10.1371/journal.pone.0009295
Smith ER, Smedberg JL, Rula ME, Xu XX (2004) Regulation of Ras-MAPK pathway mitogenic activity by restricting nuclear entry of activated MAPK in endoderm differentiation of embryonic carcinoma and stem cells. J Cell Biol 164(5):689–699. https://doi.org/10.1083/jcb.200312028
Schulte M, Köster J, Rahmann S, Schramm A (2018) Cancer evolution, mutations, and clonal selection in relapse neuroblastoma. Cell Tissue Res 372(2):263–268. https://doi.org/10.1007/s00441-018-2810-5
Acknowledgements
This work was supported by Grant-in-Aid for Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT KAKENHI Grant No. 19H03719) and by the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development, AMED (Grant No. 19ck0106332h0003). The English of this manuscript was reviewed by Dr. Brian Quinn (Editor-in-Chief, Japan Medical Communication).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanaka, T., Togashi, Y., Takeuchi, Y. et al. Immunohistochemical staining of phosphorylated-ERK in post-chemotherapeutic samples is a potential predictor of the prognosis of neuroblastoma. Pediatr Surg Int 37, 287–291 (2021). https://doi.org/10.1007/s00383-020-04806-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-020-04806-w