Abstract
Background
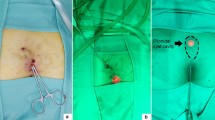
The aim of this study was to present our experience in the use of crystallized phenol (CP) to treat pediatric patients with 'simple' and complex' Pilonidal sinus disease (PSD).
Materials and methods
Patients who underwent CP treatment in between January 2015 and January 2020 were evaluated retrospectively, using prospectively collected data. The patients were divided into simple and complicated groups. The groups were assigned depending on the number of sinuses and clinical presentation. The groups were compared in terms of age, sex, number of sessions, cost analysis, body mass index (BMI), recurrence, time resolution, cosmetic results, results /improvement, and complications.
Results
This study included 54 patients: 28 (52%) girls and 26 (48%) boys. The mean number of sinuses was 2.4. Symptoms included discharge in 50 (92%) patients, and pain in 42 (78%) patients. Fifty (93%) patients experienced mild pain during the procedure, whereas four (7%) patients had moderate pain. The mean number of CP sessions was 2.9; mean numbers of CP sessions were 2.2 and 4.2 in the Simple and Complicated groups. In total, 5 of 54 patients (9%) had recurrence.At the end of treatment, therapeutic success was achieved in 49 of 54 (91%) patients: 31 of 33 (94%) patients in the Simple group and 18 of 21 patients (86%) in the complicated group. The mean treatment cost for the whole cohort was $17.40. One patient (2%) presented with moderate skin burns. Cosmesis was deemed acceptable by patients, although there was evidence of minor skin burns following the procedure.
Conclusions
The findings showed that the CP procedure was advantageous for treatment of PSD, because it was minimally invasive, cost-effective, provided good cosmesis, and had a high success rate and low complication rate. Furthermore, it did not require prior examination, and could be performed under local anesthesia. Therefore, the CP procedure may be useful as a first-line treatment option in children with PSD.


Similar content being viewed by others
References
Maurıce BA, Greenwood RK (1964) A conservative treatment of pilonidal sinus. Br J Surg 51:510–512 (PubMed PMID: 14199061)
Dogru O, Camci C, Aygen E et al (2004) Pilonidal sinus treated with crystallized phenol: an eight-year experience. Dis Colon Rectum 47:1934–1938 (PubMed PMID: 15622588)
Ates U, Ergun E, Gollu G et al (2018) Pilonidal sinus disease surgery in children: the first study to compare crystallized phenol application to primary excision and closure. J PediatrSurg 53:452–455. https://doi.org/10.1016/j.jpedsurg.2017.05.012 (PubMed PMID: 28549686)
Kayaalp C, Aydin C (2009) Review of phenol treatment in sacrococcygeal pilonidal disease. Tech Coloproctol 13:189–193 (PubMed PMID: 19655223)
Neyzi O, Gunoz H, Furman A (2008) Türkçocuklarındavücutağırlığı, boy uzunluğu, başçevresivevücutkitleindeksireferansdeğerleri. ÇocukSağlığıveHastalıklarıDergisi 51:1–14
Girgin M, Kanat BH (2014) The results of a one-time crystallized phenol application for pilonidal sinus disease. Indian J Surg 76:17–20. https://doi.org/10.1007/s12262-012-0548-y
Calikoglu I, Gulpinar K, Oztuna D, Elhan AH, Dogru O, Akyol C et al (2017) Phenol injection versus excision with open healing in pilonidal disease: a prospective randomized trial. Dis Colon Rectum 60(2):161–169. https://doi.org/10.1097/DCR.0000000000000717 (PMID: 28059912)
De Parades V, Bouchard D, Janier M et al (2013) Pilonidal sinus disease. J ViscSurg 150(4):237–247. https://doi.org/10.1016/j.jviscsurg.2013.05.006
Singh H, Agrawal M, Singh NK et al (2017) Pilonidal disease—review article. Ann Int Med Dent Res 3:18–27. https://doi.org/10.21276/aimdr.2017.3.2.SG5
Doll D, Krueger CM, Schrank S et al (2007) Timeline of recurrence after primary and secondary pilonidal sinus surgery. Dis Colon Rectum 50:1928–1934. https://doi.org/10.1007/s10350-007-9031-4
Oliveira AI, Barroso C, Osório A et al (2019) Front Pediatr 7:215. https://doi.org/10.3389/fped.2019.00215
Meinero P, Stazi A, Carbone A et al (2016) Endoscopic pilonidal sinus treatment: a prospective multicentre trial. Colorectal Dis 18(5):O164–O170. https://doi.org/10.1111/codi.13322
Meinero P, Mori L, Gasloli G (2014) Endoscopic pilonidal sinus treatment (E.P.Si.T.). Tech Coloproctol 18:389–392. https://doi.org/10.1007/s10151-013-1016-9
Emile SH, Elfeki H, ShalabyM, et al (2018) Endoscopicpilonidal sinus treatment: a systematic review and meta-analysis. SurgEndosc 32:3754–3762. https://doi.org/10.1007/s00464-018-6157-5
Iesalnieks I, Deimel S, Kienle K et al (2011) Pit-pickingoperationbeipatientenmit sinus pilonidalis. Der Chir 82:927–931. https://doi.org/10.1007/s00104-011-2077-9
Turkoglu A, Bozdag Z, Gumus M et al (2018) Comparison of crystallized phenol treatment and simple primary closure methods for pilonidal sinus disease. IntSurg 103:424–428. https://doi.org/10.9738/INTSURG-D-15-00066.1
Topuz O, Sözen S, Tükenmez M et al (2014) Crystallized phenol treatment of pilonidal disease improves quality of life. Indian J Surg 76(1):81–84. https://doi.org/10.1007/s12262-012-0610-9
Karakayali F, Karagulle E, Karabulut Z et al (2009) Unroofing and marsupialization vs rhomboid excision and Limberg flap in pilonidal disease: a prospective, randomized, clinical trial. Dis Colon Rectum. 52:496–502. https://doi.org/10.1007/DCR.0b013e31819a3ec0 (PubMed PMID: 19333052)
Yildiz MK, Ozkan E, Odabaşı HM et al (2013) Karydakis flap procedure in patients with sacrococcygeal pilonidal sinus disease: experience of a single centre in Istanbul. ScientificWorldJournal 14(2013):807027. https://doi.org/10.1155/2013/807027 (PubMed PMID: 23766710; PubMed Central PMCID: PMC3666289)
Kayaalp C, Olmez A, Aydin C et al (2010) Investigation of a one-time phenol application for pilonidal disease. Med PrincPract 19(3):212–215. https://doi.org/10.1159/000285291 (PubMed PMID: 20357505)
Girgin M, Kanat BH, Ayten R et al (2012) Minimally invasive treatment of pilonidal disease: crystallized phenol and laser depilation. Int Surg. 97(4):288–292. https://doi.org/10.9738/CC130.1 (PubMed Central PMCID: PMC3727265)
Kaymakcioglu N, Yagci G, Simsek A et al (2005) Treatment of pilonidal sinus by phenol application and factors affecting the recurrence. Tech Coloproctol 9(1):21–24 (PMID: 15868494)
Aygen E, Arslan K, Dogru O et al (2010) Crystallized phenol in nonoperative treatment of previously operated, recurrent pilonidal disease. Dis Colon Rectum 53(6):932–935. https://doi.org/10.1007/DCR.0b013e3181d8283b (PubMed PMID: 20485008)
Bayhan Z, Zeren S, Duzgun SA et al (2016) Crystallized phenol application and modified Limberg flap procedure in treatment of pilonidal sinus disease: A comparative retrospective study. Asian J Surg 39(3):172–177. https://doi.org/10.1016/j.asjsur.2015.12.007 (PubMed PMID: 26883556)
Kayaalp C, Tolan K (2015) Crystallized or Liquid Phenol Application in Pilonidal Sinus Treatment. Indian J Surg. 77(6):562–563. https://doi.org/10.1007/s12262-015-1316-6 (PubMed PMID: 26884674; PubMed Central PMCID: PMC4744226)
Dag A, Colak T, Turkmenoglu O et al (2012) Phenol procedure for pilonidal sinus disease and risk factors for treatment failure. Surgery 151(1):113–117. https://doi.org/10.1016/j.surg.2011.07.015 (PubMed PMID: 21982072)
Hegge HG, Vos GA, Patka P et al (1987) Treatment of complicated or infected pilonidal sinus disease by local application of phenol. Surgery 102:52–54
Vara-Thorbeck R, Mekinassi K, Berchid S (1990) Phenol treatment of pilonidal sinuses. ZentralblChir 115(12):777–780 (PubMed PMID: 2385975)
Al-Jaberi TMR (2001) Excision and simple primary closure of chronic pilonidal sinus. Eur J Surg 167:133–135 (PubMed PMID: 11266254)
Yuksel ME (2017) Pilonidal sinus disease can be treated with crystallized phenol using a simple three-step technique. ActaDermatovenerol Alp PannonicaAdriat 26(1):15–17 (PubMed PMID: 28352930)
Acknowledgments
The English in this document has been checked by at least two professional editors, both native speakers of English. (Textcheck reference number: 20040116, 20061120))
The study was approved by the Ethics Committee of Dicle University (Approval No. 5/3/2020-83).
Author information
Authors and Affiliations
Contributions
The CP procedure and article writing was performed by a SA. Other surgeons of the study contributed to patient follow-up and data collection, statistics, analysis and writing processes of the study.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arslan, S., Okur, M.H., Basuguy, E. et al. Crystallized phenol for treatment of pilonidal sinus disease in children: a comparative clinical study. Pediatr Surg Int 37, 807–813 (2021). https://doi.org/10.1007/s00383-020-04798-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-020-04798-7