Abstract
Background
Exposure to ionizing radiation results in cytotoxic and genotoxic effects caused mainly by the oxidative damage. In the present study, we investigated the radioprotective effect of novel antioxidant cocktail on germ cell apoptosis and spermatogenesis in rats subjected to whole body radiation (WBIR).
Methods
Adult male rats weighing 250–270 g were divided into four groups, eight rats each. Group 1 served as untreated control, group 2 received an IP single dose of antioxidant cocktail (1 ml). Group 3 was exposed to a WBIR (6 Gy). Group 4 received antioxidant cocktail before WBIR. Rats from each group were killed after 48 h. MDA levels were measured in serum (TBARS assay). Johnsen’s criteria and the number of germinal cell layers were used to categorize spermatogenesis. TUNEL assay was used to determine germ cell apoptosis. Statistical analysis was performed using one-way ANOVA test.
Results
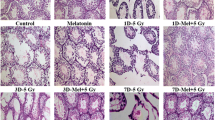
WBIR resulted in histological testicular damage (decrease in Johnsen’s criteria, p < 0.05) that was accompanied by a significant increase in germ cell apoptosis, expressed as the number of apoptotic cells per 100 tubules (AI-1 apoptotic index) and the number of positive tubules per 100 tubules (AI-2 apoptotic index). Treatment with antioxidant cocktail resulted in a significant decrease in germ cell apoptosis (33% decrease in AI-1, p < 0.05 and 34% decrease in AI-2, p < 0.05) that was accompanied by an improved spermatogenesis (increase in Johnsen’s criteria, p < 0.05).
Conclusions
In a rat model of WBIR, antioxidant treatment ameliorates oxidative stress-induced testicular damage, decreases germ cell apoptosis and improves spermatogenesis.



Similar content being viewed by others
References
Mantel F, Flentje M, Guckenberger M (2013) Stereotactic body radiation therapy in the re-irradiation situation–a review. Radiat Oncol 5:8:7–10
Sun Z, Ng KH (2012) Use of radiation in medicine in the Asia–Pacific region. Singap Med J 53(12):784–788
Jahnukainen K, Ehmcke J, Hou M, Schlatt S (2011) Testicular function and fertility preservation in male cancer patients. Best Pract Res Clin Endocrinol Metab 25(2):287–302
Marjault HB, Allemand I (2016) Consequences of irradiation on adult spermatogenesis: between infertility and hereditary risk. Mutat Res 770(Pt B):340–348
Ståhl O, Eberhard J, Jepson K (2006) Sperm DNA integrity in testicular cancer patients. Hum Reprod 21:3199–3205
Shalet SM, Tsatsoulis A, Whitehead E, Read G (1989) Vulnerability of the human Leydig cell to radiation damage is dependent upon age. J Endocrinol 120:161–165
Limon-Pacheco J, Gonsebatt ME (2009) The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res 674:137–147
Cadet J, Mouret S, Ravanat JL, Douki T (2012) Photoinduced damage to cellular DNA: direct and photosensitized reactions. Photochem Photobiol 88:1048–1065
Miller DC, Peron SE, Keck RW, Kropp KA (1990) Effects of hypothermia on testicular ischemia. J Urol 143:1046–1048
Johnsen SG (1970) Testicular biopsy score count—a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1:2–25
Tang FR, Loke WK, Khoo BC (2017) Low-dose or low-dose-rate ionizing radiation-induced bioeffects in animal models. J Radiat Res 2017 58(2):165–182
Harms-Ringdahl M, Nicotera P, Radford IR (1996) Radiation induced apoptosis. Mutat Res 366:171–179
Lavin MF (1998) Radiation-induced cell death and its implications in human disease. Res Probl Cell Differ 24:213–232
Modi DN, Sane S, Bhartiya D (2003) Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol Hum Reprod 9:219–225
Bartke A (1995) Apoptosis of male germ cells, a generalized or cell type-specific phenomenon. Endocrinology 136:3–4
Kimura M, Itoh N, Takagi S, Sasao T, Takahashi A, Masumori N, Tsukamoto T (2003) Balance of apoptosis and proliferation of germ cells related to spermatogenesis in aged men. J Androl 24:185–191
Hikim AP, Lue Y, Yamamoto CM, Vera Y, Rodriguez S, Yen PH, Soeng K, Wang C, Swerdloff RS (2003) Key apoptotic pathways for heat-induced programmed germ cell death in the testis. Endocrinology 144:3167–3175
Sukhotnik I, Voskoboinik K, Lurie M, Bejar Y, Coran AG, Mogilner JG (2009) Involvement of the bax and bcl-2 system in the induction of germ cell apoptosis is correlated with the time of reperfusion after testicular ischemia in a rat model. Fertil Steril 92(4):1466–1469
Löbrich M, Rief N, Kühne M, Heckmann M, Fleckenstein J, Rübe C, Uder M (2005) In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci USA 102:8984–8989
Gutteridge JMC (1994) Biological origin of free radicals, and mechanisms of antioxidant protection. Chem Biol Interact 91:133–140
Machlin LJ, Bendich A (1987) Free radical tissue damage: protective role of antioxidant nutrients. FASEB J 1(6):441–445
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab 111(4):524–527
Shaban NZ, Helmy MH, El-Kersh MA, Mahmoud BF (2003) Effects of Bacillus thuringiensis toxin on hepatic lipid peroxidation and free-radical scavengers in rats given alpha-tocopherol or acetylsalicylate. Comp Biochem Physiol C Toxicol Pharmacol 135:405–414
Batista LF, Kaina B, Meneghini R, Menck CF (2009) How DNA lesions are turned into powerful killing structures: insights from UV-induced apoptosis. Mutat Res 681:197–208
Borrás C, Gómez-Cabrera MC, Viña J (2011) The dual role of p53: DNA protection and antioxidant. Free Radic Res 45:643–652
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sukhotnik, I., Nativ, O., Ben-Shahar, Y. et al. Antioxidant treatment ameliorates germ cell apoptosis induced by a high-dose ionizing irradiation in rats. Pediatr Surg Int 35, 137–143 (2019). https://doi.org/10.1007/s00383-018-4385-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-018-4385-3