Abstract
Purpose
Pulmonary hypoplasia (PH), characterized by alveolar immaturity, is one of the leading causes of respiratory insufficiency in newborns with congenital diaphragmatic hernia (CDH). Leptin (Lep) and its receptor (Lep-R) play an important role in fetal lung growth by stimulating alveolar differentiation and maturation. Lep and Lep-R are strongly expressed by alveolar cells during the saccular stage of fetal lung development. Lep-deficient mice exhibit decreased alveolarization with reduced pulmonary surfactant phospholipid synthesis, similar to human and nitrofen-induced PH. Prenatal administration of all-trans retinoic acid (ATRA) has been shown to stimulate alveolarization in nitrofen-induced PH. Recent studies have demonstrated that Lep and Lep-R expression in developing lungs is regulated by ATRA. We hypothesized that prenatal treatment with ATRA increases pulmonary Lep and Lep-R expression in the nitrofen model of CDH-associated PH.
Methods
Time-mated rats received either 100 mg nitrofen or vehicle via oral-gastric lavage on embryonic day 9.5 (E9.5). Control and nitrofen-exposed dams were randomly assigned to either intraperitoneal ATRA (5 mg/kg/d) or placebo administration on E18.5, E19.5 and E20.5. Fetal lungs were harvested on E21.5, and divided into Control+Placebo, Control+ATRA, Nitrofen+Placebo and Nitrofen+ATRA. Alveolarization was assessed using stereo- and morphometric analysis techniques. Surfactant phospholipid synthesis was analyzed by labeling for surfactant protein B (SP-B). Pulmonary gene expression levels of Lep and Lep-R were determined using quantitative real-time polymerase chain reaction. Immunohistochemical staining for Lep and Lep-R was performed to evaluate alveolar protein expression and localization.
Results
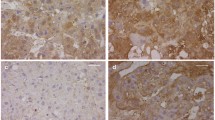
In vivo administration of ATRA resulted in significantly increased lung-to-body weight ratio with enhanced radial alveolar count and decreased mean linear intercept compared to placebo treatment. Immunofluorescence analysis demonstrated markedly increased pulmonary SP-B expression in Nitrofen+ATRA compared to Nitrofen+Placebo. Relative mRNA expression of Lep and Lep-R was significantly increased in Nitrofen+ATRA compared to Nitrofen+Placebo. Lep and Lep-R immunoreactivity was markedly increased in interstitial and alveolar epithelial cells of Nitrofen+ATRA compared to Nitrofen+Placebo.
Conclusion
Increased Lep and Lep-R expression after prenatal administration of ATRA in nitrofen-induced PH suggests that ATRA may have therapeutic potential in attenuating CDH-associated PH by stimulating alveolarization and de novo surfactant production.



Similar content being viewed by others
References
Gallot D, Boda C, Ughetto S et al (2007) Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry-based study. Ultrasound Obstet Gynecol 29(3):276–283
Loane M, Dolk H, Kelly A et al (2011) Paper 4: EUROCAT statistical monitoring: identification and investigation of ten year trends of congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol 91(Suppl 1):S31–S43
Slavotinek AM (2014) The genetics of common disorders—Congenital diaphragmatic hernia. Eur J Med Genet 57(8):418–423
Keijzer R, Puri P (2010) Congenital diaphragmatic hernia. Semin Pediatr Surg 19(3):180–185
Peetsold MG, Heij HA, Kneepkens CM et al (2009) The long-term follow-up of patients with a congenital diaphragmatic hernia: a broad spectrum of morbidity. Pediatr Surg Int 25(1):1–17
Rocha G, Azevedo I, Pinto JC et al (2012) Follow-up of the survivors of congenital diaphragmatic hernia. Early Hum Dev 88(4):255–258
Chiu PP (2014) New insights into congenital diaphragmatic hernia—a surgeon’s introduction to CDH animal models. Front Pediatr 2:36
Noble BR, Babiuk RP, Clugston RD et al (2007) Mechanisms of action of the congenital diaphragmatic hernia-inducing teratogen nitrofen. Am J Physiol Lung Cell Mol Physiol 293(4):L1079–L1087
van Loenhout RB, Tibboel D, Post M et al (2009) Congenital diaphragmatic hernia: comparison of animal models and relevance to the human situation. Neonatology 96(3):137–149
Montedonico S, Nakazawa N, Puri P (2008) Congenital diaphragmatic hernia and retinoids: searching for an etiology. Pediatr Surg Int 24(7):755–761
Clugston RD, Zhang W, Alvarez S et al (2010) Understanding abnormal retinoid signaling as a causative mechanism in congenital diaphragmatic hernia. Am J Respir Cell Mol Biol 42(3):276–285
Doi T, Sugimoto K, Puri P (2009) Up-regulation of COUP-TFII gene expression in the nitrofen-induced hypoplastic lung. J Pediatr Surg 44(2):321–324
Montedonico S, Nakazawa N, Puri P (2006) Retinoic acid rescues lung hypoplasia in nitrofen-induced hypoplastic foetal rat lung explants. Pediatr Surg Int 22(1):2–8
Montedonico S, Sugimoto K, Felle P et al (2008) Prenatal treatment with retinoic acid promotes pulmonary alveologenesis in the nitrofen model of congenital diaphragmatic hernia. J Pediatr Surg 43(3):500–507
Torday JS, Sun H, Wang L et al (2002) Leptin mediates the parathyroid hormone-related protein paracrine stimulation of fetal lung maturation. Am J Physiol Lung Cell Mol Physiol 282(3):L405–L410
Henson MC, Swan KF, Edwards DE et al (2004) Leptin receptor expression in fetal lung increases in late gestation in the baboon: a model for human pregnancy. Reproduction 127(1):87–94
Tankersley C, Kleeberger S, Russ B et al (1996) Modified control of breathing in genetically obese (ob/ob) mice. J Appl Physiol 81(2):716–723
McGowan SE, Harvey CS, Jackson SK (1995) Retinoids, retinoic acid receptors, and cytoplasmic retinoid binding proteins in perinatal rat lung fibroblasts. Am J Physiol 269(4 Pt 1):L463–L472
Randell SH, Mercer RR, Young SL (1989) Postnatal growth of pulmonary acini and alveoli in normal and oxygen-exposed rats studied by serial section reconstructions. Am J Anat 186(1):55–68
Herriges M, Morrisey EE (2014) Lung development: orchestrating the generation and regeneration of a complex organ. Development 141(3):502–513
Alfanso LF, Arnaiz A, Alvarez FJ et al (1996) Lung hypoplasia and surfactant system immaturity induced in the fetal rat by prenatal exposure to nitrofen. Biol Neonatol 69(2):94–100
Utsuki T, Hashizume K, Iwamori M (2001) Impaired spreading of surfactant phospholipids in the lungs of newborn rats with pulmonary hypoplasia as a model of congenital diaphragmatic hernia induced by nitrofen. Biochim Biophys Acta 1531(1–2):90–98
Simon DM, Mariani TJ (2007) Role of PPARs and retinoid X receptors in the regulation of lung maturation and development. PPAR Res 2007:91240
Sugimoto K, Takayasu H, Nakazawa N et al (2008) Prenatal treatment with retinoic acid accelerates type 1 alveolar cell proliferation of the hypoplastic lung in the nitrofen model of congenital diaphragmatic hernia. J Pediatr Surg 43(2):367–372
Huang K, Rabold R, Abston E et al (2008) Effects of leptin deficiency on postnatal lung development in mice. J Appl Physiol 105(1):249–259
Bergen HT, Cherlet TC, Manuel P et al (2002) Identification of leptin receptors in lung and isolated fetal type II cells. Am J Respir Cell Mol Biol 27(1):71–77
Chen H, Zhang JP, Huang H et al (2013) Leptin promotes fetal lung maturity and upregulates SP-A expression in pulmonary alveoli type-II epithelial cells involving TTF-1 activation. PLoS One 8(7):e69297
Kirwin SM, Bhandari V, Dimatteo D et al (2006) Leptin enhances lung maturity in the fetal rat. Pediatr Res 60(2):200–204
Massaro D, Massaro GD (2010) Lung development, lung function, and retinoids. N Engl J Med 362(19):1829–1831
Chytil F (1996) Retinoids in lung development. FASEB J 10(9):986–992
Desai A, Kartono F, Del Rosso JQ (2007) Systemic retinoid therapy: a status report on optimal use and safety of long-term therapy. Dermatol Clin 25(2):185–193
Valappil S, Kurkar M, Howell R (2007) Outcome of pregnancy in women treated with all-trans retinoic acid; a case report and review of literature. Hematology 12(5):415–418
Acknowledgments
This research was supported by the National Children’s Research Centre and the Children’s Medical and Research Foundation.
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friedmacher, F., Hofmann, A.D., Takahashi, T. et al. Prenatal administration of all-trans retinoic acid upregulates leptin signaling in hypoplastic rat lungs with experimental congenital diaphragmatic hernia. Pediatr Surg Int 30, 1183–1190 (2014). https://doi.org/10.1007/s00383-014-3605-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-014-3605-8