Abstract
Purpose
Pleuroperitoneal folds (PPFs) represent the only source of muscle precursors cells (MPCs) in the primordial diaphragm. However, the exact pathogenesis of malformed PPFs and congenital diaphragmatic hernia (CDH) remains unclear. The muscle-specific transcription factor myogenin plays a key role during development and muscularization of the fetal diaphragm. Although myogenin knockout mice lack skeletal muscle fibers, the diaphragmatic musculature is intact without any defects. It has further been demonstrated that proliferation and differentiation of MPCs in PPFs and developing diaphragms are normal in rodent CDH models. We hypothesized that myogenin gene expression is not altered in malformed PPFs, developing diaphragms and diaphragmatic musculature in the nitrofen-induced CDH model.
Methods
Pregnant rats were exposed to nitrofen or vehicle on gestational day 9 (D9). Fetuses were harvested during PPF formation (D13), diaphragmatic development (D14-15) and muscularization (D18-21). Fetal PPFs, developing diaphragms and diaphragmatic musculature were dissected and divided into nitrofen and control groups. Myogenin mRNA levels were analyzed by quantitative real-time polymerase chain reaction, while immunohistochemistry was performed to investigate myogenin protein expression and distribution.
Results
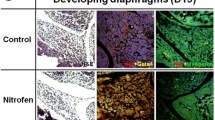
Relative mRNA expression of myogenin was not significant different in PPFs (0.30 ± 0.09 vs. 0.48 ± 0.09; P = 0.37), developing diaphragms (1.25 ± 0.29 vs. 1.60 ± 0.32; P=0.53) and diaphragmatic musculature (1.08 ± 0.24 vs. 1.59 ± 0.20; P = 0.15) of nitrofen-exposed fetuses compared to controls. Myogenin immunoreactivity was not altered in the muscular components of malformed PPFs, developing diaphragms and diaphragmatic musculature of nitrofen-exposed fetuses compared to controls.
Conclusion
Myogenin gene expression is not altered in PPFs, developing diaphragms and diaphragmatic musculature in the nitrofen-induced CDH model, thus suggesting that diaphragmatic defects in this model develop independent of myogenic processes.

Similar content being viewed by others
References
Keijzer R, Puri P (2010) Congenital diaphragmatic hernia. Semin Pediatr Surg 19(3):180–185
Clugston RD, Klattig J, Englert C, Clagett-Dame M, Martinovic J, Benachi A, Greer JJ (2006) Teratogen-induced, dietary and genetic models of congenital diaphragmatic hernia share a common mechanism of pathogenesis. Am J Pathol 169(5):1541–1549
Babiuk RP, Zhang W, Clugston R, Allan DW, Greer JJ (2003) Embryological origins and development of the rat diaphragm. J Comp Neurol 455(4):477–487
Greer JJ (2013) Current concepts on the pathogenesis and etiology of congenital diaphragmatic hernia. Respir Physiol Neurobiol 189:232–240
Montedonico S, Nakazawa N, Puri P (2008) Congenital diaphragmatic hernia and retinoids: searching for an etiology. Pediatr Surg Int 24(7):755–761
Noble BR, Babiuk RP, Clugston RD, Underhill TM, Sun H, Kawaguchi R, Walfish PG, Blomhoff R, Gundersen TE, Greer JJ (2007) Mechanisms of action of the congenital diaphragmatic hernia-inducing teratogen nitrofen. Am J Physiol Lung Cell Mol Physiol 293(4):L1079–L1087
Clugston RD, Greer JJ (2007) Diaphragm development and congenital diaphragmatic hernia. Semin Pediatr Surg 16(2):94–100
Wright WE, Sassoon DA, Lin VK (1989) Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56(4):607–617
Olson EN, Klein WH (1994) bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev 8(1):1–8
Goncalves FL, Oliveira FS, Schmidt AF, Pereira LA, Gallindo RM, Figueira RL, Sbragia L (2013) Effect of nitrofen in the final stages of development of the diaphragm muscle in rats. Acta Cir Bras 28(Suppl 1):13–18
Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH (1993) Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364(6437):501–506
Tseng BS, Cavin ST, Booth FW, Olson EN, Marin MC, McDonnell TJ, Butler IJ (2000) Pulmonary hypoplasia in the myogenin null mouse embryo. Am J Respir Cell Mol Biol 22(3):304–315
Babiuk RP, Greer JJ (2002) Diaphragm defects occur in a CDH hernia model independently of myogenesis and lung formation. Am J Physiol Lung Cell Mol Physiol 283(6):L1310–L1314
Mayer S, Metzger R, Kluth D (2011) The embryology of the diaphragm. Semin Pediatr Surg 20(3):161–169
Clugston RD, Zhang W, Greer JJ (2010) Early development of the primordial mammalian diaphragm and cellular mechanisms of nitrofen-induced congenital diaphragmatic hernia. Birth Defects Res A Clin Mol Teratol 88(1):15–24
Merrell AJ, Kardon G (2013) Development of the diaphragm—a skeletal muscle essential for mammalian respiration. FEBS J 280(17):4026–4035
Hinterberger TJ, Sassoon DA, Rhodes SJ, Konieczny SF (1991) Expression of the muscle regulatory factor MRF4 during somite and skeletal myofiber development. Dev Biol 147(1):144–156
Buckingham M (2007) Skeletal muscle progenitor cells and the role of Pax genes. C R Biol 330(6–7):530–533
Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M (2006) Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol 172(1):91–102
Lopes Fda S, Carvalho RF, Campos GE, Sugizaki MM, Padovani CR, Nogueira CR, Cicogna AC, Pai-Silva MD (2008) Down-regulation of MyoD gene expression in rat diaphragm muscle with heart failure. Int J Exp Pathol 89(3):216–222
Ropka-Molik K, Eckert R, Piorkowska K (2011) The expression pattern of myogenic regulatory factors MyoD, Myf6 and Pax7 in postnatal porcine skeletal muscles. Gene Expr Patterns: GEP 11(1–2):79–83
Kassar-Duchossoy L, Gayraud-Morel B, Gomes D, Rocancourt D, Buckingham M, Shinin V, Tajbakhsh S (2004) Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature 431(7007):466–471
Li J, Liu KC, Jin F, Lu MM, Epstein JA (1999) Transgenic rescue of congenital heart disease and spina bifida in Splotch mice. Development 126(11):2495–2503
Gosemann JH, Doi T, Kutasy B, Friedmacher F, Dingemann J, Puri P (2012) Pax3 gene expression is not altered during diaphragmatic development in nitrofen-induced congenital diaphragmatic hernia. J Pediatr Surg 47(6):1067–1071
Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R (1993) MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75(7):1351–1359
Inanlou MR, Kablar B (2003) Abnormal development of the diaphragm in mdx:MyoD−/− (9th) embryos leads to pulmonary hypoplasia. Int J Dev Biol 47(5):363–371
Dingemann J, Doi T, Ruttenstock E, Puri P (2011) The role of primary myogenic regulatory factors in the developing diaphragmatic muscle in the nitrofen-induced diaphragmatic hernia. Pediatr Surg Int 27(6):579–582
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, T., Friedmacher, F., Takahashi, H. et al. Myogenin gene expression is not altered in the developing diaphragm of nitrofen-induced congenital diaphragmatic hernia. Pediatr Surg Int 30, 901–906 (2014). https://doi.org/10.1007/s00383-014-3557-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-014-3557-z