Abstract
Background
The neonatal surgical patient is threatened by exuberant inflammatory reactions. Neonatal macrophages are key players in this process. We investigated the ability of neonatal macrophages to initiate a local inflammatory reaction upon exposure to different bacterial or viral ligands to toll-like receptors (TLRs).
Methods
Peritoneal wash outs from neonatal (<24 h) and adult (42 days) C57BL/6J mice were gained by peritoneal lavages. In a first set of experiments, macrophages were purified and stimulated for 6 h by four different TLR ligands. mRNA was extracted for transcriptome analysis. In a second set of experiments, lipopolysaccharide was applied into peritoneal cavities. After 6 h of incubation, the cellular composition of the inflamed cavities was evaluated by cytological staining as well as chipcytometry.
Results
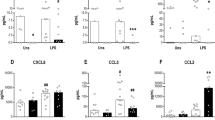
Neonatal murine peritoneal macrophages differed significantly in the expression of pro- and anti-chemotactic genes. Functional assignment of these genes revealed enhanced chemotactic potential of neonatal macrophages and was confirmed by a higher influx of pro-inflammatory cells into neonatal peritoneal cavities.
Conclusion
Neonatal peritoneal macrophages demonstrated an enhanced chemotactic potential upon stimulation with four TLR ligands. This was associated with an increased influx of inflammatory cells to the peritoneal cavity. This might contribute to the strong inflammatory responses of neonates and preterms.



Similar content being viewed by others
References
Lawn JE, Cousens S, Zupan J (2005) 4 million neonatal deaths: when? Where? Why? The Lancet 365:891–900
Afrazi A, Sodhi CP, Richardson W et al (2011) New insights into the pathogenesis and treatment of necrotizing enterocolitis: toll-like receptors and beyond. Pediatr Res 69:183–188
Maródi L (2006) Neonatal innate immunity to infectious agents. Infect Immun 74:1999–2006
Lewis DB, Wilson CB (2006) Developmental immunology and role of host defenses in fetal and neonatal susceptibility to infection. In: Infectious diseases of the fetus and newborn infant, Chapter 4, Sixth edn. W.B. Saunders, Philadelphia, pp 87–210
Levy O, Zarember KA, Roy RM et al (2004) Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-a induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol 173:4627–4634
Winterberg T, Yu Y, Vieten G et al (2012) Immature phenotype of neonatal peritoneal myeloid cells is not affected by birth or intestinal microbial colonization. XXV international symposium on paediatric surgical research, London, 21 September 2012
Mantovani A, Sica A, Sozzani S et al (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Medzhitov R, Horng T (2009) Transcriptional control of the inflammatory response. Nat Rev Immunol 9:692–703
Szatmary Z (2012) Molecular biology of toll-like receptors. Gen Physiol Biophys 31:357–366
Schulz C, Perdiguero EG, Chorro L et al (2012) A lineage of myeloid cells independent of myb and hematopoietic stem cells. Science 335:86–90
Hashimoto D, Chow A, Noizat C et al (2013) Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38:792–804
Hennig C, Adams N, Hansen G (2009) A versatile platform for comprehensive chip-based explorative cytometry. Cytometry Part A 75A:362–370
Cailhier JF, Sawatzky DA, Kipari T et al (2006) Resident pleural macrophages are key orchestrators of neutrophil recruitment in pleural inflammation. Am J Respir Crit Care Med 173:540–547
Beck-Schimmer B, Schwendener R, Pasch T et al (2005) Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respir Res 6:61
Cailhier JF, Partolina M, Vuthoori S et al (2005) Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol 174:2336–2342
Wynn TA, Chawla A, Pollard JW (2013) Macrophage biology in development, homeostasis and disease. Nature 496:445–455
Philbin VJ, Levy O (2009) Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res 65:98R–105R
Corbett NP, Blimkie D, Ho KC et al (2010) Ontogeny of toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One 5(11):e15041
Liao S, Yeh K, Lai S et al (2013) Maturation of toll-like receptor 1–4 responsiveness during early life. Early Hum Dev 89:473–478
Jiang H, Van De Ven C, Satwani P et al (2004) Differential gene expression patterns by oligonucleotide microarray of basal versus lipopolysaccharide-activated monocytes from cord blood versus adult peripheral blood. J Immunol 172:5870–5879
Belge K, Dayyani F, Horelt A et al (2002) The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol 168:3536–3542
Soehnlein O, Lindbom L (2010) Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 10:427–439
Fox SE, Lu W, Maheshwari A et al (2005) The effects and comparative differences of neutrophil specific chemokines on neutrophil chemotaxis of the neonate. Cytokine 29:135–140
Christensen RD (1989) Hematopoiesis in the fetus and neonate. Pediatr Res 26:531–535
Zhang X, Goncalves R, Mosser DM (2008) The isolation and characterization of murine macrophages. Curr Protoc Immunol 83:14.1.1–14.1.14
Schofield ZV, Woodruff TM, Halai R, et al. (2013) Neutrophils—a key component of ischemia reperfusion injury. Shock 40:463–470
Acknowledgments
This work was funded by the DFG (Deutsche Forschungsgemeinschaft, KU 2759/1-1). Microarray data used or referred to in this publication were generated by the Research Core Unit Transcriptomics of the Hannover Medical School. Special thanks got to Kerstin Wiesner for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winterberg, T., Vieten, G., Feldmann, L. et al. Neonatal murine macrophages show enhanced chemotactic capacity upon toll-like receptor stimulation. Pediatr Surg Int 30, 159–164 (2014). https://doi.org/10.1007/s00383-013-3457-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-013-3457-7