Abstract
Background
Growing evidence suggests that the Wnt/β-catenin signaling cascade is implicated in the control of stem cell activity, cell proliferation, lineage commitment, and cell survival during normal development and tissue regeneration of the gastrointestinal epithelium. The roles of this signaling cascade in stimulation of cell proliferation after massive small bowel resection are unknown. The purpose of this study was to evaluate the role of Wnt/β-catenin signaling during late stages of intestinal adaptation in a rat model of short bowel syndrome (SBS).
Methods
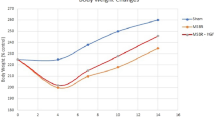
Male rats were divided into two groups: sham rats underwent bowel transection and SBS rats underwent a 75 % bowel resection. Parameters of intestinal adaptation, enterocyte proliferation and apoptosis were determined 2 weeks after operation. Illumina’s digital gene expression analysis was used to determine Wnt/β-catenin signaling gene expression profiling. Twelve Wnt/β-catenin-related genes and β-catenin protein expression were determined using real-time PCR, western blotting and immunohistochemistry.
Results
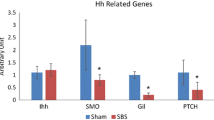
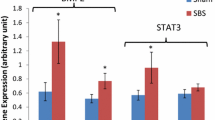
From the total number of 20,000 probes, 20 genes related to Wnt/β-catenin signaling were investigated. From these genes, seven genes were found to be up-regulated and eight genes to be down-regulated in SBS vs. sham animals with a relative change in gene expression level of 20 % or more. From 12 genes determined by real-time PCR, nine genes were down-regulated in SBS rats compared to control animals including target gene c-Myc. SBS rats also showed a significant decrease in β-catenin protein compared to control animals.
Conclusion
Two weeks following massive bowel resection in rats, Wnt/β-catenin signaling pathway is inhibited. In addition, it appears that cell differentiation rather than proliferation is most important in the late stages of intestinal adaptation.



Similar content being viewed by others
References
Coran AG, Spivak D, Teitelbaum DH (1999) An analysis of the morbidity and mortality of short bowel syndrome in the pediatric age group. Eur J Pediatr Surg 9:228–230
O’Brien DP, Nelson LA, Huang FS, Warner BW (2001) Intestinal adaptation: structure, function, and regulation. Sem Pediatr Surg 10:55–64
Martin GR, Wallace LE, Hartmann B, Holst JJ, Demchyshyn L, Toney K, Sigalet DL (2005) Nutrient-stimulated GLP-2 release and crypt cell proliferation in experimental short bowel syndrome. Am J Physiol Gastrointest Liver Physiol 288:G431–G438
De Santa Barbara P, van den Brink GR, Roberts DJ (2003) Development and differentiation of the intestinal epithelium. Cell Mol Life Sci 2003(60):1322–1323
Haegebarth A, Clevers H (2009) Wnt signaling, Lgr5, and stem cells in the intestine. Am J Pathol 174:15–21
Van de Wetering M, Sancho E, Verweij C (2002) The β-catenin/TCF-4 complex impoves a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241–250
Katoh M, Katoh M (2007) WNT signaling pathway and stem cell signaling network. Clin Cancer Res 13:4042–4045
Turashvili G, Bouchal J, Burkadze G, Kolar Z (2006) Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology 73:213–223
Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M (2004) PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 430:93–98
Lu W, Yamamoto V, Ortega B, Baltimore D (2004) Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119:97–108
Bernal NP, Stehr W, Zhang Y, Profitt S, Erwin CR, Warner BW (2005) Evidence for active Wnt signaling during postresection intestinal adaptation. J Pediatr Surg 40:1025–1029
Wang L, Tang Y, Rubin DC, Levin MS (2007) Chronically administered retinoic acid has trophic effects in the rat small intestine and promotes adaptation in a resection model of short bowel syndrome. Am J Physiol Gastrointest Liver Physiol 292:G1559–G1569
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127:469–480
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26
Eisenmann DM (2005) Wnt signaling. In: WormBook (ed.) The C. elegans Research Community WormBook. doi:10.1895/wormbook.1.7.1
Hirata A, Utikal J, Yamashita S, Aoki H, Watanabe A, Yamamoto T, Okano H, Bardeesy N, Kunisada T, Ushijima T, Hara A, Jaenisch R, Hochedlinger K, Yamada Y (2013) Dose-dependent roles for canonical Wnt signalling in de novo crypt formation and cell cycle properties of the colonic epithelium. Development 1(140):66–75
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 18(111):241–250
Horvay K, Abud HE (2013) Regulation of intestinal stem cells by wnt and notch signalling. Adv Exp Med Biol 786:175–186
Farin HF, Van Es JH, Clevers H (2012) Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143:1518–1529
Liang H, Chen Q, Coles AH (2003) Wnt5a inhibits B-cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell 4:349–360
He TC, Sparks AB, Rago C (1998) Identification of c-Myc as a target of the APC pathway. Science 281:1509–1512
Shtutman M, Zhurinsky J, Simcha I (1999) The cyclinD1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 96:5522–5527
Zhang T, Nanney LB, Peeler MO (1997) Decreased transforming growth factor beta type II receptor expression in intestinal adenomas from Min/+ mice is associated with increased cyclin D1andcyclin-dependent kinase 4 expression. Cancer Res 57:1638–1643
Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T (2002) β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111:251–263
Acknowledgments
This work was supported by Atkins Medical Research Fund (2013, No. 2016688).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sukhotnik, I., Roitburt, A., Pollak, Y. et al. Wnt/β-catenin signaling cascade down-regulation following massive small bowel resection in a rat. Pediatr Surg Int 30, 173–180 (2014). https://doi.org/10.1007/s00383-013-3447-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-013-3447-9