Abstract
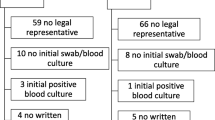
Static electricity within sterile packaging may result in bacterial contamination of central venous catheters (CVCs) prior to insertion. To prevent this, some surgeons inject saline into the pack before opening it. This trial was designed to determine the effect of this procedure. A double blind randomised controlled trial of 47 CVCs comparing injection of 2 ml of sterile saline into the pack prior to opening with no injection was performed. Five centimetre lengths cut from the tip of the catheter before and after subcutaneous tunnelling were sent for microbiological culture. Eight catheters (17%) showed evidence of bacterial contamination prior to insertion into the vein. Two (4.2%) were contaminated prior to tunnelling and seven (14.9%) afterwards. One catheter was contaminated before and after tunnelling. All but one of the contaminating bacteria were coagulase negative staphylococci. There was no significant difference in the contamination rate between catheters from packs that had been injected (5/25) and those that had not (3/22), P=0.56. Just under one-fifth of the catheters were contaminated with bacteria prior to insertion into the vein but this was not influenced by prior injection of saline into the pack. We conclude that there is no evidence to support the practice of injecting the catheter pack prior to opening.


Similar content being viewed by others
References
O’grady NP, Alexander M, Dellinger EP et al (2002) Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 30:476–489
Allen JE, Henshaw DL, Wynne H, Ross F, Oakhill A (2003) Static electric charge may contribute to infections in bone marrow transplant wards. J Hosp Infect 54:80–81
Cozanitis DA, Ojajarvi J, Makela P (1988) Antistatic treatment for reducing airborne contamination of insulating materials in intensive care. Acta Anaesthesiol Scand 32:343–346
Colomb V, Fabeiro M, Dabbas M, Goulet O, Merckx J, Ricour C (2000) Central venous catheter-related infections in children on long-term home parenteral nutrition: incidence and risk factors. Clin Nutr 19:355–359
Das I, Philpott C, George RH (1997) Central venous catheter-related septicaemia in paediatric cancer patients. J Hosp Infect 36:67–76
Domm JA, Hudson MG, Janco RL (2003) Complications of central venous access devices in paediatric haemophilia patients. Haemophilia 9:50–56
Tarantino MD, Lail A, Donfield SM et al (2003) Surveillance of infectious complications associated with central venous access devices in children with haemophilia. Haemophilia 9:588–592
Shaul DB, Scheer B, Rokhsar S et al (1998) Risk factors for early infection of central venous catheters in pediatric patients. J Am Coll Surg 186:654–658
Acknowledgements
Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from R and D funding received from the NHS Executive.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hall, N.J., Hartley, J., Ade-Ajayi, N. et al. Bacterial contamination of central venous catheters during insertion: a double blind randomised controlled trial. Ped Surgery Int 21, 507–511 (2005). https://doi.org/10.1007/s00383-005-1478-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-005-1478-6