Abstract
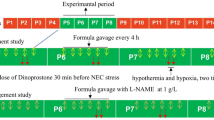
The etiology of intussusception (IN) remains largely obscure. In lipopolysaccharide (LPS)-induced IN, an experimental model in mice, IN is considered to be the consequence of altered intestinal motility as a result of increased nitric oxide (NO) along various inflammatory mediators. These could be decreased via cyclooxygenase (COX) inhibition by indomethacin. N-ω-nitro-L-arginine methyl ester (L-NAME) inhibits nitric oxide synthase (NOS) and NO production. Indomethacin is known to prevent IN; however, the reason is unknown. In this study we aimed to determine the role of NO, the effects of inhibition of its production by L-NAME and indomethacin, and whether preventive effects of indomethacin on LPS-induced IN were related to NO inhibition. A total of 113 mice were divided into seven groups. In the control group (n=6), no procedure was done. In the sham group (n=6), 1 ml saline was given; in the indomethacin group (n=6), 10 mg/kg of indomethacin was given; and in the LPS group (n=30), 12 mg/kg of LPS was administered intraperitoneally (IP). In the LPS+indomethacin group (n=32), 10 mg/kg of indomethacin was administered IP simultaneously with 12 mg/kg of LPS. In the L-NAME group (n=6), 20 mg/kg of L-NAME was administered subcutaneously. In LPS+L-NAME group (n=27), 20 mg/kg of L-NAME was administered subcutaneously with 12 mg/kg of LPS IP. All animals were laparotomized 6 h following injections. Existence of IN was noted and blood specimens were obtained. NO was quantified by measurements of nitrite and nitrate, obtaining a total of NO metabolites (NOx). The results were compared using the Mann–Whitney U-test and Spearman correlation test. A value of p<0.05 was considered significant. A total of 17 mice (one in control, 10 in LPS, four in LPS+indomethacin, and two in LPS+L-NAME groups) were excluded from the study because of death or insufficient blood collection. LPS (12 mg/kg, IP) induced IN at a rate of 30% (n=6) in the LPS group. Mean NOx levels were statistically higher in the LPS group (186.67±20.06) compared with other groups (p<0.05). Mean NOx levels were significantly higher in the group of mice with IN than in those without in the LPS group of this study (295.46±16.42, 140.05±15.44, respectively, p<0.05). The mean NOx levels were statistically lower in the LPS+L-NAME(23.94±3.39) group than the LPS+indomethacin (106.77±24.54) group, with no IN detected in neither of these two groups. Increased NOx levels induced by LPS correlated well with the occurrence of IN, and decreasing these levels via COX inhibition by indomethacin or NOS inhibition by L-NAME totally prevented IN from forming in this study. By these observations, it could be concluded that NO is probably involved in the pathophysiology of IN in this experimental model of LPS-induced IN.
Similar content being viewed by others

References
Willetts IE, Kite P, Barclay GR, et al. (2001) Endotoxin, cytokines and lipid peroxides in children with intussusception. Br J Surg 88:878–883
Masne AL, Lortat-Jacob S, Sayegh N, et al. (1999) Intussusception in infants and children: feasibility of ambulatory management. Eur J Pediatr 158:707–710
Fallat ME (2000) Intussusception. In: Ashcraft KW, Murphy JP, Sharp RJ, et al. (eds) Pediatric surgery. WB Saunders, Philadelphia, pp 518–526
Young DG (1998) Intussusception. In: O’Neil JA, Rowe MI, Grosfel JL, et al. (eds) Pediatric surgery. Mosby, St. Louis, pp 1185–1198
Nissan A, Zhang JM, Lin Z, et al. (1997) The contribution of inflammatory mediators and nitric oxide to lipopolysaccharide-induced intussusception in mice. J Surg Res 69:205–207
Hellström PM, Al-Saffar A, Ljung T, et al. (1997) Endotoxin actions on myoelectric activity, transit and neuropeptides in the gut role of nitric oxide. Dig Dis Sci 42(8):1640–1651
Matsuda H, Li Y, Yoshikawa M (2000) Roles of endogenous prostaglandins and nitric oxide in inhibitions of gastric emptying and accelerations of gastrointestinal transit by escins Ia, Ib, IIa and II b in mice. Life Sci 66(3):PL41–PL46
Cullen JJ, Doty RC, Ephgrave KS, et al. (1999) Changes in intestinal transit and absorption during endotoxemia are dose dependent. J Surg Res 81:81–86
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5(1):62–71
Hocking MP, McCoy DM, Vogel SB, et al. (1991) Antiperistaltic and isoperistaltic intussusception associated with abnormal motility after Roux-en-Y gastric bypass: a case report. Surgery 110:109–112
Zhong L, Patrizia C, Nissan A, et al. (1998) Bacterial wall lipopolysaccharide as a cause of intussusception in mice. J Pediatr Gastroenterol Nutr 27(3):301–305
Cullen JJ, Caropreso DK, Ephgrave KS, et al. (1997) The effect of endotoxin on canine jejunal motility and transit. J Surg Res 67:54–57
Rees DD, Celleck S, Palmer RMJ, et al. (1990) Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun 173:541–547
Salvemini D, Misko TP, Masferrer JL, et al. (1993) Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA 90:7240–7244
Vivancos M, Moreno JJ (2002) Role of Ca(2+)-independent phospholipase A(2) and cyclooxygenase/lipoxygenase pathways in the nitric oxide production by murine macrophages stimulated by lipopolysaccharide Nitric Oxide 6:255–262
Gaillard T, Mülsch A, Busse R, et al. (1991) Regulation of nitric oxide production by stimulated rat Kuppfer cells. Pathobiology 59:280–283
Marotta P, Sautebin L, DiRosa M (1992) Modulation of the induction of nitric oxide synthase by eicosanoid in the murine macrophage cell line J 774. Br J Pharmacol 107:640–641
Chen L, Salafranca MN, Mehta JL (1997) Cyclooxygenase inhibition decreases nitric oxide synthase activity in human platelets. Am J Physiol 273: H1854–H1859
Acknowledgments
This study was sponsored by the Scientific Research Foundation of Gazi University (01/2003-06).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Türkyılmaz, Z., Karabulut, R., Gülen, Ş. et al. Role of nitric oxide and cyclooxygenase pathway in lipopolysaccharide-induced intussusception. Ped Surgery Int 20, 598–601 (2004). https://doi.org/10.1007/s00383-004-1239-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-004-1239-y