Abstract
Purpose
Coherence analysis in electroencephalography (EEG) allows measurement of the degree of consistency of amplitude between pairs of electrodes. Theoretically, disconnective epilepsy surgery should decrease coherence between corresponding areas. The study aimed to evaluate postoperative changes in interhemispheric coherence values after corpus callosotomy (CC).
Methods
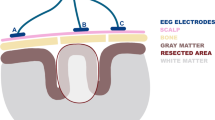
Non-lesional, drug-resistant, generalized epilepsy patients who underwent total CC were retrospectively collected. To evaluate coherence, we divided the scalp interictal EEG into “baseline” and “discharge” states after excluding periods with artifacts. Interhemispheric coherence values were obtained between eight pairs of symmetrically opposite scalp electrodes in six different frequency bands. We analyzed both pre- and postoperative EEG sessions and calculated the percentage of difference (POD) in coherence values.
Results
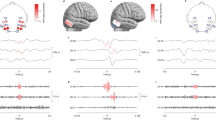
We collected 13 patients and analyzed 2496 interhemispheric coherence values. Preoperative coherence values differed significantly between baseline and discharge states (p = 0.0003), but postoperative values did not (p = 0.11). For baseline state, coherence values were decreased after CC and median POD was − 22.3% (p < 0.0001). Delta frequency showed the most decreased POD (-44.3%, p = 0.0009). Median POD was lowest in the Fp1-Fp2 pair of electrodes. For discharge state, coherence values were decreased after CC and median POD was − 24.7% (p < 0.0001). Delta frequency again showed the most decreased POD (-55.9%, p = 0.0016). Median POD was lowest in the F7-F8 pair.
Conclusion
After total CC, interhemispheric coherence decreased significantly in both baseline and discharge states. The most decreased frequency band was the delta band, which may be used as a representative frequency band in future studies.




Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
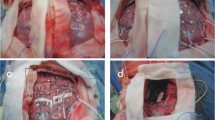
Uda T, Kunihiro N, Umaba R, Koh S, Kawashima T, Ikeda S, Ishimoto K, Goto T (2021) Surgical aspects of corpus callosotomy. Brain Sci 11(12):1608. https://doi.org/10.3390/brainsci11121608. PMID: 34942910
Thohar Arifin M, Muttaqin Z, Bakhtiar Y et al (2020) Seizure outcomes in patients with complete versus anterior corpus callosotomy: analysis of outcome. Int J Gen Med 13:105–110
Sunaga S, Shimizu H, Sugano H (2009) Long-term follow-up of seizure outcomes after corpus callosotomy. Seizure 18(2):124–128
Graham D, Tisdall MM, Gill D (2016) Corpus callosotomy outcomes in pediatric patients: a systematic review. Epilepsia 57(7):1053–1068
Chan AY, Rolston JD, Lee B et al (2018) Rates and predictors of seizure outcome after corpus callosotomy for drug-resistant epilepsy: a meta-analysis. J Neurosurg 130(4):1193–1202
Koh S, Uda T, Kunihiro N et al (2022) Disconnection surgery to cure or palliate medically intractable epileptic spasms: a retrospective study. J Neurosurg Pediatr 29(6):693–699
Baba H, Toda K, Ono T et al (2018) Surgical and developmental outcomes of corpus callosotomy for West syndrome in patients without MRI lesions. Epilepsia 59(12):2231–2239
Velagapudi L, Matias CM, Ambrose TM et al (2021) Alternate seizure spread with agenesis of the corpus callosum. J Epilepsy Res 11(1):100–105
Jeong G-U, Kim H, Lim BC et al (2011) Predictive value of electroencephalography for seizure outcome following corpus callosotomy in children. J Epilepsy Res 1(2):65–70
Kanai S, Oguri M, Okanishi T et al (2019) Symmetry of ictal slow waves may predict the outcomes of corpus callosotomy for epileptic spasms. Sci Rep 9(1):19733
Oguri M, Okanishi T, Kanai S, Baba S, Nishimura M, Ogo K, Himoto T, Okanari K, Maegaki Y, Enoki H, Fujimoto A (2020) Phase lag analyses on ictal scalp electroencephalography may predict outcomes of corpus callosotomy for epileptic spasms. Front Neurol 11:576087. https://doi.org/10.3389/fneur.2020.576087
Okumura E, Iwasaki M, Sakuraba R et al (2013) Time-varying inter-hemispheric coherence during corpus callosotomy. Clin Neurophysiol 124(11):2091–2100
Srinivasan R, Winter WR, Ding J et al (2007) EEG and MEG coherence: measures of functional connectivity at distinct spatial scales of neocortical dynamics. J Neurosci Methods 166(1):41–52
Greenblatt RE, Pflieger ME, Ossadtchi AE (2012) Connectivity measures applied to human brain electrophysiological data. J Neurosci Methods 207(1):1–16
Bowyer SM (2016) Coherence a measure of the brain networks: past and present. Neuropsychiatric Electrophysiol 2(1):1
Montplaisir J, Nielsen T, Côté J et al (1990) Interhemispheric EEG coherence before and after partial callosotomy. Clin Electroencephalogr 21(1):42–47
Matsuhashi A, Matsuo T, Kumada S (2022) Incremental changes in interhemispheric functional connectivity after two-stage corpus callosotomy in a patient with subcortical band heterotopia. Epilepsy Behav Rep 18:100525
Oguni H, Olivier A, Andermann F et al (1991) Anterior callosotomy in the treatment of medically intractable epilepsies: a study of 43 patients with a mean follow-up of 39 months. Ann Neurol 30(3):357–364
Lee C-C, Hung S-C, Chen Y-H et al (2022) Structural connectivity in children after total corpus callosotomy. Epilepsy Res 182:106908
Johnston JM, Vaishnavi SN, Smyth MD et al (2008) Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci 28(25):6453–6458
Uddin LQ, Mooshagian E, Zaidel E et al (2008) Residual functional connectivity in the split-brain revealed with resting-state functional MRI. Neuroreport 19(7):703–709
Pizoli CE, Shah MN, Snyder AZ et al (2011) Resting-state activity in development and maintenance of normal brain function. Proc Natl Acad Sci USA 108(28):11638–11643
Roland JL, Snyder AZ, Hacker CD et al (2017) On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proc Natl Acad Sci USA 114(50):13278–13283
Liang J-G, Kim N-Y, Ko A et al (2018) Changes in functional brain network topology after successful and unsuccessful corpus callosotomy for lennox-gastaut syndrome. Sci Rep 8(1):3414
Acknowledgements
A draw figure was illustrated by Dr. Ariya Mongkolporn.
Funding
This work was supported by JSPS KAKENHI Grant Number JP22K09212.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: Yindeedej, Uda. Acquisition of data: Uda, Nishijima, Inoue, Kuki, Fukuoka, Nukui, Okazaki. Patient care: Inoue, Kuki, Fukuoka, Nukui, Okazaki, Kunihiro, Umaba. Analysis and interpretation of data: Yindeedej, Uda. Drafting of the manuscript: Yindeedej. Critical revision: Uda. Review of the submitted version of the manuscript: All authors. Approval of the final version: Uda. Study supervision: Goto.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Osaka City General Hospital and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
The patient consented to the procedure and written informed consent was obtained.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yindeedej, V., Uda, T., Nishijima, S. et al. Changes in interhemispheric coherence after total corpus callosotomy: a scalp EEG study in children with non-lesional generalized epilepsy. Childs Nerv Syst (2024). https://doi.org/10.1007/s00381-024-06435-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00381-024-06435-3