Abstract
Purpose
It has been commonly accepted for a long time that the cerebrospinal fluid (CSF) drains into arachnoid granulations from the subarachnoid space to the dural venous sinus unidirectionally. However, recently, periventricular capillaries and lymphatic concepts have been introduced. The CSF moves along the perivascular space and drains into the capillary vessels or meningeal lymphatic tissues. CSF is involved in removing brain waste out of the brain. In this study, we investigated the outflow mechanism of substances in the CSF from the brain.
Methods
We investigated the movement of CSF by injection of gold colloid conjugates (2, 40, and 200 nm) into the lateral ventricles of mouse fetuses and evaluated the deposition by silver stain with tissue transparency and electron microcopy. Cadaverine was also injected into the lateral ventricle to determine its movement tract.
Results
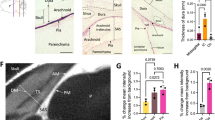
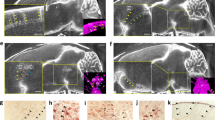
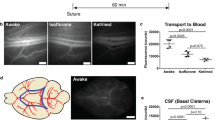
The gold particle deposition was mainly observed in the frontal skull base. Electron microscopic study showed that the gold particle deposition was observed on the choroid plexus and ependyma in the lateral ventricle and also red blood cells in the heart and liver. Two-nanometer particles were exclusively observed in the liver. Cadaverine injection study demonstrated that cadaverine was observed at the extracranial frontal skull base, choroid plexus, ependymal surface, and perivascular area in the brain white matter.
Conclusion
The particles in the CSF were shown to move from the brain to the frontal skull base and also into the blood stream through the choroid plexus in the fetus. The outflow of particles in the CSF may be regulated by molecular size. This new information will contribute to the prevention of brain degeneration due to brain waste deposition.




Similar content being viewed by others
References
Oresković D, Klarica M (2010) The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev 64:241–262. https://doi.org/10.1016/j.brainresrev.2010.04.006
Shokohi R, Nabiuni M, Irian S, Miyan JA (2018) In vitro effects of wistar rat prenatal and postnatal cerebrospinal fluid on neural differentiation and proliferation of mesenchymal stromal cells derived from bone marrow. Cell J 19:537–544. https://doi.org/10.22074/cellj.2018.4130
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4:147ra111. https://doi.org/10.1126/scitranslmed.3003748
Iliff JJ, Goldman SA, Nedergaard M (2015) Implications of the discovery of brain lymphatic pathways. Lancet Neurol 14:977–979. https://doi.org/10.1016/s1474-4422(15)00221-5
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76:845–861. https://doi.org/10.1002/ana.24271
Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, Overall CC, Acton ST, Kipnis J (2018) Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560:185–191. https://doi.org/10.1038/s41586-018-0368-8
Weed LH (1914) Studies on cerebro-spinal fluid. No. III: the pathways of escape from the Subarachnoid Spaces with particular reference to the Arachnoid Villi. J Med Res 31:51–91
Welch K, Pollay M (1961) Perfusion of particles through arachnoid villi of the monkey. Am J Physiol 201:651–654. https://doi.org/10.1152/ajplegacy.1961.201.4.651
McComb JG (1983) Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg 59:369–383. https://doi.org/10.3171/jns.1983.59.3.0369
Gómez DG, DiBenedetto AT, Pavese AM, Firpo A, Hershan DB, Potts DG (1982) Development of arachnoid villi and granulations in man. Acta Anat (Basel) 111:247–258. https://doi.org/10.1159/000145473
Yamada S, Miyazaki M, Kanazawa H, Higashi M, Morohoshi Y, Bluml S, McComb JG (2008) Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology 249:644–652. https://doi.org/10.1148/radiol.2492071985
Beck DJ, Russell DS (1946) Experiments on thrombosis of the superior longitudinal sinus. J Neurosurg 3:337–347. https://doi.org/10.3171/jns.1946.3.4.0337
Guthrie TC, Dunbar HS, Karpell B (1970) Ventricular size and chronic increased intracranial venous pressure in the dog. J Neurosurg 33:407–414. https://doi.org/10.3171/jns.1970.33.4.0407
Hammock MK, Milhorat TH, Earle K, Di Chiro G (1971) Vein of Galen ligation in the primate. Angiographic, gross, and light microscopic evaluation. J Neurosurg 34:77–83. https://doi.org/10.3171/jns.1971.34.1.0077
Gangemi M, Maiuri F, Naddeo M, Godano U, Mascari C, Broggi G, Ferroli P (2008) Endoscopic third ventriculostomy in idiopathic normal pressure hydrocephalus: an Italian multicenter study. Neurosurgery 63:62–67; discussion 67–69. https://doi.org/10.1227/01.Neu.0000335071.37943.40
Hailong F, Guangfu H, Haibin T, Hong P, Yong C, Weidong L, Dongdong Z (2008) Endoscopic third ventriculostomy in the management of communicating hydrocephalus: a preliminary study. J Neurosurg 109:923–930. https://doi.org/10.3171/jns/2008/109/11/0923
Bulat M, Lupret V, Orešković D, Klarica M (2008) Transventricular and transpial absorption of cerebrospinal fluid into cerebral microvessels. Coll Antropol 31(Suppl 3):43–50
Bulat M, Klarica M (2011) Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res Rev 65:99–112. https://doi.org/10.1016/j.brainresrev.2010.08.002
Klarica M, Radoš M, Orešković D (2019) The movement of cerebrospinal fluid and its relationship with substances behavior in cerebrospinal and interstitial fluid. Neuroscience 414:28–48. https://doi.org/10.1016/j.neuroscience.2019.06.032
Akai T, Hatta T, Shimada H, Mizuki K, Kudo N, Hatta T, Otani H (2018) Extracranial outflow of particles solved in cerebrospinal fluid: fluorescein injection study. Congenit Anom (Kyoto) 58:93–98. https://doi.org/10.1111/cga.12257
Yamada M, Hatta T, Otani H (2008) Mouse exo utero development system: protocol and troubleshooting. Congenit Anom (Kyoto) 48:183–187. https://doi.org/10.1111/j.1741-4520.2008.00203.x
Sakata-Haga H, Uchishiba M, Shimada H, Tsukada T, Mitani M, Arikawa T, Shoji H, Hatta T (2018) A rapid and nondestructive protocol for whole-mount bone staining of small fish and Xenopus. Sci Rep 8:7453. https://doi.org/10.1038/s41598-018-25836-4
Getachew D, Kaneda R, Saeki Y, Matsumoto A, Otani H (2021) Morphologic changes in the cytoskeleton and adhesion apparatus during the conversion from pseudostratified single columnar to stratified squamous epithelium in the developing mouse esophagus. Congenit Anom (Kyoto) 61:14–24. https://doi.org/10.1111/cga.12389
Yamamoto S, Yoshino I, Shimazaki T, Murohashi M, Hevner RF, Lax I, Okano H, Shibuya M, Schlessinger J, Gotoh N (2005) Essential role of Shp2-binding sites on FRS2alpha for corticogenesis and for FGF2-dependent proliferation of neural progenitor cells. Proc Natl Acad Sci U S A 102:15983–15988. https://doi.org/10.1073/pnas.0507961102
Földi M, Gellért A, Kozma M, Poberai M, Zoltán OT, Csanda E (1966) New contributions to the anatomical connections of the brain and the lymphatic system. Acta Anat (Basel) 64:498–505. https://doi.org/10.1159/000142849
Zhang ET, Richards HK, Kida S, Weller RO (1992) Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropathol 83:233–239. https://doi.org/10.1007/bf00296784
Kida S, Pantazis A, Weller RO (1993) CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19:480–488. https://doi.org/10.1111/j.1365-2990.1993.tb00476.x
Hsu M, Rayasam A, Kijak JA, Choi YH, Harding JS, Marcus SA, Karpus WJ, Sandor M, Fabry Z (2019) Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat Commun 10:229. https://doi.org/10.1038/s41467-018-08163-0
Johnston M, Papaiconomou C (2002) Cerebrospinal fluid transport: a lymphatic perspective. News Physiol Sci 17:227–230. https://doi.org/10.1152/nips.01400.2002
Koh L, Zakharov A, Nagra G, Armstrong D, Friendship R, Johnston M (2006) Development of cerebrospinal fluid absorption sites in the pig and rat: connections between the subarachnoid space and lymphatic vessels in the olfactory turbinates. Anat Embryol (Berl) 211:335–344. https://doi.org/10.1007/s00429-006-0085-1
Kwon S, Janssen CF, Velasquez FC, Sevick-Muraca EM (2017) Fluorescence imaging of lymphatic outflow of cerebrospinal fluid in mice. J Immunol Methods 449:37–43. https://doi.org/10.1016/j.jim.2017.06.010
Ma Q, Ineichen BV, Detmar M, Proulx ST (2017) Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 8:1434. https://doi.org/10.1038/s41467-017-01484-6
Nagra G, Johnston MG (2007) Impact of ageing on lymphatic cerebrospinal fluid absorption in the rat. Neuropathol Appl Neurobiol 33:684–691. https://doi.org/10.1111/j.1365-2990.2007.00857.x
Badaut J, Lasbennes F, Magistretti PJ, Regli L (2002) Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab 22:367–378. https://doi.org/10.1097/00004647-200204000-00001
Owler BK, Pitham T, Wang D (2010) Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal Fluid Res 7:15. https://doi.org/10.1186/1743-8454-7-15
Paul L, Madan M, Rammling M, Chigurupati S, Chan SL, Pattisapu JV (2011) Expression of aquaporin 1 and 4 in a congenital hydrocephalus rat model. Neurosurgery 68:462–473. https://doi.org/10.1227/NEU.0b013e3182011860
Milhorat TH, Mosher MB, Hammock MK, Murphy CF (1970) Evidence for choroid-plexus absorption in hydrocephalus. N Engl J Med 283:286–289. https://doi.org/10.1056/nejm197008062830604
Hashimoto PH (1988) Tracer in cisternal cerebrospinal fluid is soon detected in choroid plexus capillaries. Brain Res 440:149–152. https://doi.org/10.1016/0006-8993(88)91167-5
Orešković D, Radoš M, Klarica M (2017) Role of choroid plexus in cerebrospinal fluid hydrodynamics. Neuroscience 354:69–87. https://doi.org/10.1016/j.neuroscience.2017.04.025
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212:991–999. https://doi.org/10.1084/jem.20142290
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341. https://doi.org/10.1038/nature14432
Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J (2017) Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127:3210–3219. https://doi.org/10.1172/jci90603
Da Mesquita S, Fu Z, Kipnis J (2018) The meningeal lymphatic system: a new player in neurophysiology. Neuron 100:375–388. https://doi.org/10.1016/j.neuron.2018.09.022
Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA (1985) Evidence for a “paravascular” fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326:47–63. https://doi.org/10.1016/0006-8993(85)91383-6
Furukawa M, Shimoda H, Kajiwara T, Kato S, Yanagisawa S (2008) Topographic study on nerve-associated lymphatic vessels in the murine craniofacial region by immunohistochemistry and electron microscopy. Biomed Res 29:289–296. https://doi.org/10.2220/biomedres.29.289
Funding
This work was supported by a grant, JSPS KAKENHI (18K08963), Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Contributions
Conception and design: T. Akai, T. Hatta, S. Kuroda. Acquisition of data: T. Akai, S. Yamamoto, T. Hatta, H. Sakata-Haga, H. Otani. Analysis and interpretation of data: T. Akai, T. Hatta, S. Yamamoto. Critically revising the article: T. Akai, T. Hatta, S. Yamamoto, S. Kuroda. Reviewed submitted version of the manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: T. Akai.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest concerning the materials or methods used in this study or the findings specified in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akai, T., Hatta, T., Sakata-Haga, H. et al. Cerebrospinal fluid may flow out from the brain through the frontal skull base and choroid plexus: a gold colloid and cadaverine injection study in mouse fetus. Childs Nerv Syst 37, 3013–3020 (2021). https://doi.org/10.1007/s00381-021-05253-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05253-1