Abstract
Purpose
Intracranial germ cell tumors (IGCTs) account for 8–15% of brain tumors in children in Eastern Asia and typically occur at the age of puberty. Recently, adjuvant therapy has been modified to ameliorate post-treatment complications such as cognitive dysfunction, in patients with IGCT. However, endocrine problems remain to be resolved. There is reluctance to use growth hormone (GH) at an early stage after treatment for IGCT because of the risk of tumor recurrence; however, GH replacement must be administered before the onset of puberty in children with short stature. There is little guidance on this issue due to a lack of literature on the risks of GH replacement therapy in patients with IGCT. This study aimed to evaluate the safety of GH replacement.
Methods
In this retrospective study, 6 patients with IGCT who were under the age of 18 years when they started GH replacement therapy were reviewed. Histopathological analysis and/or analysis of tumor markers was used to confirm a diagnosis.
Results
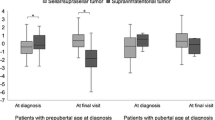
All six cases who underwent GH replacement therapy showed no recurrence. GH replacement therapy was administered in 5 patients for short stature and all achieved a height within ± 2.0 standard deviation.
Conclusion
GH replacement for children with IGCT was safe in our case series. Placental alkaline phosphatase levels in cerebrospinal fluid can be used to facilitate the decision on when to initiate GH replacement.

Similar content being viewed by others
References
Liu B, Arakawa Y, Yokogawa R, Tokunaga S, Terada Y, Murata D, Matsui Y, Fujimoto KI, Fukui N, Tanji M, Mineharu Y, Minamiguchi S, Miyamoto S (2018) PD-1/PD-L1 expression in a series of intracranial germinoma and its association with Foxp3+ and CD8+ infiltrating lymphocytes. PLoS One 13:e0194594
Phi JH, Wang KC, Kim SK (2018) Intracranial germ cell tumor in the molecular era. J Korean Neurosurg Soc 61:333–342
Odagiri K, Omura M, Hata M, Aida N, Niwa T, Ogino I, Kigasawa H, Ito S, Adachi M, Inoue T (2012) Treatment outcomes, growth height, and neuroendocrine functions in patients with intracranial germ cell tumors treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys 84:632–638
Packer RJ, Cohen BH, Cooney K (2000) Intracranial germ cell tumors. Oncologist 5:312–320
Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, Seto T (1997) Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg 86:446–455
Liu APY, Hastings C, Wu S, Bass JK, Heitzer AM, Ashford J, Vestal R, Hoehn ME, Ghazwani Y, Acharya S, Conklin HM, Boop F, Merchant TE, Gajjar A, Qaddoumi I (2019) Treatment burden and long-term health deficits of patients with low-grade gliomas or glioneuronal tumors diagnosed during the first year of life. Cancer 125:1163–1175
Bogarin R, Steinbok P (2009) Growth hormone treatment and risk of recurrence or progression of brain tumors in children: a review. Childs Nerv Syst 25:273–279
Sklar CA, Mertens AC, Mitby P, Occhiogrosso G, Qin J, Heller G, Yasui Y, Robison LL (2002) Risk of disease recurrence and second neoplasms in survivors of childhood cancer treated with growth hormone: a report from the childhood cancer survivor study. J Clin Endocrinol Metab 87:3136–3141
Gleeson HK, Stoeter R, Ogilvy-Stuart AL, Gattamaneni HR, Brennan BM, Shalet SM (2003) Improvements in final height over 25 years in growth hormone (GH)-deficient childhood survivors of brain tumors receiving GH replacement. J Clin Endocrinol Metab 88:3682–3689
Geffner M, Lundberg M, Koltowska-Haggstrom M, Abs R, Verhelst J, Erfurth EM, Kendall-Taylor P, Price DA, Jonsson P, Bakker B (2004) Changes in height, weight, and body mass index in children with craniopharyngioma after three years of growth hormone therapy: analysis of KIGS (Pfizer International Growth Database). J Clin Endocrinol Metab 89:5435–5440
Indini A, Schiavello E, Biassoni V, Bergamaschi L, Magni MC, Puma N, Chiaravalli S, Pallotti F, Seregni E, Diletto B, Pecori E, Gandola L, Poggi G, Massimino M (2017) Long-term safety of growth hormone replacement therapy after childhood medulloblastoma and PNET: it is time to set aside old concerns. J Neuro-Oncol 131:349–357
Darendeliler F, Karagiannis G, Wilton P, Ranke MB, Albertsson-Wikland K, Anthony Price D, On Behalf Of The Kigs International B (2006) Recurrence of brain tumours in patients treated with growth hormone: analysis of KIGS (Pfizer International Growth Database). Acta Paediatr 95:1284–1290
Swerdlow AJ, Reddingius RE, Higgins CD, Spoudeas HA, Phipps K, Qiao Z, Ryder WD, Brada M, Hayward RD, Brook CG, Hindmarsh PC, Shalet SM (2000) Growth hormone treatment of children with brain tumors and risk of tumor recurrence. J Clin Endocrinol Metab 85:4444–4449
Friend KE, Radinsky R, McCutcheon IE (1999) Growth hormone receptor expression and function in meningiomas: effect of a specific receptor antagonist. J Neurosurg 91:93–99
Sklar CA, Antal Z, Chemaitilly W, Cohen LE, Follin C, Meacham LR, Murad MH (2018) Hypothalamic-pituitary and growth disorders in survivors of childhood cancer: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 103:2761–2784
Child CJ, Conroy D, Zimmermann AG, Woodmansee WW, Erfurth EM, Robison LL (2015) Incidence of primary cancers and intracranial tumour recurrences in GH-treated and untreated adult hypopituitary patients: analyses from the hypopituitary control and complications study. Eur J Endocrinol 172:779–790
Yuen KCJ, Biller BMK, Radovick S, Carmichael JD, Jasim S, Pantalone KM, Hoffman AR (2019) American Association of Clinical Endocrinologists and American College of endocrinology guidelines for management of growth hormone deficiency in adults and patients transitioning from pediatric to adult care. Endocr Pract 25:1191–1232
Aihara Y, Watanabe S, Amano K, Komatsu K, Chiba K, Imanaka K, Hori T, Ohba T, Dairoku H, Okada Y, Kubo O, Kawamata T (2018) Placental alkaline phosphatase levels in cerebrospinal fluid can have a decisive role in the differential diagnosis of intracranial germ cell tumors. J Neurosurg:1–8
Richmond EJ, Rogol AD (2008) Growth hormone deficiency in children. Pituitary 11:115–120
Ogata T, Tanaka T, Kagami M (2007) Target height and target range for Japanese children: revisited. Clin Pediatr Endocrinol 16:85–87
Cameron N (1993) The Tanner-Whitehouse II skeletal maturity method: rationale and applicability. Clin Pediatr Endocrinol 2:9–18
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Pfaffle R (2015) Hormone replacement therapy in children: the use of growth hormone and IGF-I. Best Pract Res Clin Endocrinol Metab 29:339–352
Li Q, You C, Liu L, Rao Z, Sima X, Zhou L, Xu J (2013) Craniopharyngioma cell growth is promoted by growth hormone (GH) and is inhibited by tamoxifen: involvement of growth hormone receptor (GHR) and IGF-1 receptor (IGF-1R). J Clin Neurosci 20:153–157
Cohen P, Clemmons DR, Rosenfeld RG (2000) Does the GH-IGF axis play a role in cancer pathogenesis? Growth Hormon IGF Res 10:297–305
Karavitaki N, Warner JT, Marland A, Shine B, Ryan F, Arnold J, Turner HE, Wass JA (2006) GH replacement does not increase the risk of recurrence in patients with craniopharyngioma. Clin Endocrinol 64:556–560
Alotaibi NM, Noormohamed N, Cote DJ, Alharthi S, Doucette J, Zaidi HA, Mekary RA, Smith TR (2018) Physiologic growth hormone-replacement therapy and craniopharyngioma recurrence in pediatric patients: a meta-analysis. World Neurosurg 109:487–496 e481
Niu DM, Guo WY, Pan HC, Wong TT (2002) Rapid enlargement of a residual craniopharyngioma during short-term growth hormone replacement. Childs Nerv Syst 18:164–165
Olsson DS, Buchfelder M, Wiendieck K, Kremenevskaja N, Bengtsson BA, Jakobsson KE, Jarfelt M, Johannsson G, Nilsson AG (2012) Tumour recurrence and enlargement in patients with craniopharyngioma with and without GH replacement therapy during more than 10 years of follow-up. Eur J Endocrinol 166:1061–1068
Calaminus G, Kortmann R, Worch J, Nicholson JC, Alapetite C, Garre ML, Patte C, Ricardi U, Saran F, Frappaz D (2013) SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro-Oncology 15:788–796
Muller HL, Gebhardt U, Schroder S, Pohl F, Kortmann RD, Faldum A, Zwiener I, Warmuth-Metz M, Pietsch T, Calaminus G, Kolb R, Wiegand C, Sorensen N, study committee of K (2010) Analyses of treatment variables for patients with childhood craniopharyngioma--results of the multicenter prospective trial KRANIOPHARYNGEOM 2000 after three years of follow-up. Horm Res Paediatr 73:175–180
Gupta S, Bi WL, Giantini Larsen A, Al-Abdulmohsen S, Abedalthagafi M, Dunn IF (2018) Craniopharyngioma: a roadmap for scientific translation. Neurosurg Focus 44:E12
Acknowledgments
We thank Dr. Kostadin Karagiozov, Mr. David Hang, and the staff of the Department of Neurosurgery at Tokyo Women’s Medical University for their guidance and assistance in preparing this paper.
Funding
This study was supported by research funds from the Department of Neurosurgery at Tokyo Women’s Medical University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kentaro Chiba, Yasuo Aihara, and Takakazu Kawamata. The first draft of the manuscript was written by Kentaro Chiba and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This study was approved by the Human Investigation Committee at our hospital and performed in accordance with the Declaration of Helsinki. Because of the retrospective nature of the study, the institutional review board waived the requirement for informed consent. To protect patient privacy, we removed all identifiers from our records upon completion of our analyses.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chiba, K., Aihara, Y. & Kawamata, T. Clinical experience of growth hormone replacement for pediatric intracranial germ cell tumor. Childs Nerv Syst 36, 1755–1760 (2020). https://doi.org/10.1007/s00381-020-04549-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04549-y