Abstract
Purpose
Intrathecal baclofen administration is commonly used in the treatment of children’s spasticity. In general, candidates for baclofen pump are patients with spastic form of cerebral palsy. Intrathecal baclofen in the treatment of spasticity due to a metabolic disorder is rarely reported.
Methods
Authors report on an 11-year-old boy with mucopolysaccharidosis type II (Hunter syndrome) with progressive stiffness and contractures followed by profound loss of joint movement range and tiptoe walking pattern. Patient was indicated for baclofen test with subsequent pump insertion and continuous intrathecal baclofen administration.
Results
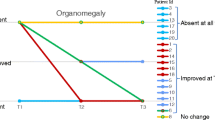
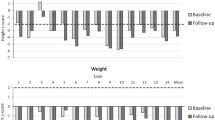
Postoperatively, patient was gradually set to current baclofen dose of 250 μg/day. At mentioned dose, we observed not only increased active and passive range of movements and facilitation in fine motor skills, but also better walking pattern.
Conclusions
Despite intrathecal baclofen administration in patients with spasticity related to mucopolysaccharidosis type II is not widely reported, we consider it as feasible treatment. To emphasize, enzyme replacement therapy is the primary treatment, and improvement is attributed to both enzyme substitution and intrathecal baclofen therapy.
Similar content being viewed by others
References
Ammar A, Ughratdar I, Sivakumar G, Vloeberghs MH (2012) Intrathecal baclofen therapy—how we do it. J Neurosurg Pediatr 10:439–444. https://doi.org/10.3171/2012.8.PEDS11475
Chu ML, Sala DA, Weiner HL (2001) Intrathecal baclofen in X-linked adrenoleukodystrophy. Pediatr Neurol 24:156–158
Da Silva EM, Strufaldi MW, Andriolo RB, Silva LA (2016) Enzyme replacement therapy with idursulfase for mucopolysaccharidosis type II (Hunter syndrome). Cochrane Database Syst Rev 2:CD008185. https://doi.org/10.1002/14651858.CD008185.pub4
Dan B, Motta F, Vles JS, Vloeberghs M, Becher JG, Eunson P, Gautheron V, Lutjen S, Mall V, Pascual-Pascual SI, Pauwels P, Roste GK (2010) Consensus on the appropriate use of intrathecal baclofen (ITB) therapy in paediatric spasticity. Eur J Paediatr Neurol 14:19–28. https://doi.org/10.1016/j.ejpn.2009.05.002
Hlavatá A et al (2012) Our experience with diagnosis and treatment of mucopolysaccharidosis Naše skúsenosti s diagnostikou a liečbou mukopolysacharidóz. Neurológia pre prax. Abstrakty : <46.> Slovensko-české dni detskej neurológie XXII. Bratislavské postgraduálne dni detskej neurológie: 17. – 20121 9. May, Bratislava: [suplement], 13, pp 22–23. ISSN 1337–4451
Ingale H, Ughratdar I, Muquit S, Moussa AA, Vloeberghs MH (2016) Selective dorsal rhizotomy as an alternative to intrathecal baclofen pump replacement in GMFCS grades 4 and 5 children. Childs Nerv Syst 32(2):321–325
Jurecka A, Krumina Z, Zuber Z, Rozdzynska-Swiatkowska A, Kloska A, Czartoryska B, Tylki-Szymanska A (2012) Mucopolysaccharidosis type II in females and response to enzyme replacement therapy. Am J Med Genet A 158A:450–454. https://doi.org/10.1002/ajmg.a.34415
Kvascevicius R, Lapteva O, Kesiene J, Vaitkus A, Mikulenaite L, Raugalas R, Sipylaite J, Rocka S, Juocevicius A (2016) Intrathecal baclofen therapy for the treatment of spasticity in Lithuania. J Neurol Surg A Cent Eur Neurosurg 78:281–285
Muenzer J, Hendriksz CJ, Fan Z, Vijayaraghavan S, Perry V, Santra S, Solanki GA, Mascelli MA, Pan L, Wang N, Sciarappa K, Barbier AJ (2016) A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet Med 18:73–81. https://doi.org/10.1038/gim.2015.36
Noh H, Lee JI (2014) Current and potential therapeutic strategies for mucopolysaccharidoses. J Clin Pharm Ther 39:215–224. https://doi.org/10.1111/jcpt.12136
Pratini NR, Neufeld JA (2014) Intrathecal baclofen therapy after liver transplant in a patient with Crigler-Najjar syndrome. PM R 6:196–198. https://doi.org/10.1016/j.pmrj.2013.09.004
Roujeau T, Di Rocco F, Zérah M (2008) Transition from childhood to adulthood and management of spasticity. Neurochirurgie 54(5):618–620
Scarpa M, Almassy Z, Beck M, Bodamer O, Bruce IA, De Meirleir L, Guffon N, Guillen-Navarro E, Hensman P, Jones S, Kamin W, Kampmann C, Lampe C, Lavery CA, Teles EL, Link B, Lund AM, Malm G, Pitz S, Rothera M, Stewart C, Tylki-Szymanska A, van der Ploeg A, Walker R, Zeman J, Wraith JE, Hunter Syndrome Europena Expert C (2011) Mucopolysaccharidosis type II: European recommendations for the diagnosis and multidisciplinary management of a rare disease. Orphanet J Rare Dis 6:72. https://doi.org/10.1186/1750-1172-6-72
Sivakumar G, Yap Y, Tsegaye M, Vloeberghs M (2010) Intrathecal baclofen therapy for spasticity of cerebral origin—does the position of the intrathecal catheter matter? Child’s Nerv Syst 26:1097–1102. https://doi.org/10.1007/s00381-010-1124-z
Sohn YB, Cho SY, Park SW, Kim SJ, Ko AR, Kwon EK, Han SJ, Jin DK (2013) Phase I/II clinical trial of enzyme replacement therapy with idursulfase beta in patients with mucopolysaccharidosis II (Hunter syndrome). Orphanet J Rare Dis 8:42. https://doi.org/10.1186/1750-1172-8-42
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Horn, F., Petrík, M., Dúbravová, D. et al. Intrathecal baclofen in mucopolysaccharidosis type II (Hunter syndrome): case report. Childs Nerv Syst 34, 2325–2327 (2018). https://doi.org/10.1007/s00381-018-3857-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-018-3857-z