Abstract
Purpose
This study aimed to determine relationships between cerebral blood flow and neurodevelopmental outcomes in children with moderate to severe traumatic brain injury (TBI).
Methods
Children with TBI, a Glasgow Coma Score of 8–12, and abnormal brain imaging were enrolled prospectively. Cerebral blood flow velocity (CBFV) was assessed within 24 h of trauma and daily thereafter through death, discharge, or hospital day 8, whichever came first. Twelve months from injury, participants completed neurodevelopmental testing.
Results
Sixty-nine patients were enrolled. Low flow velocities (< 2 SD below age/gender normal) were found in 6% (n = 4). No patient with a single low CBFV measurement had a good neurologic outcome (Pediatric Glasgow Outcome Scale (GOS-E Peds) ≤ 4)). Normal flow velocities (± 2 SD around age/gender normal) were seen in 43% of participants (n = 30). High flow velocities (> 2 SD above age and gender normal with a Lindegaard ratio (LR) < 3) were identified in 23% of children (n = 16), and vasospasm (> 2 SD above age/gender normal with LR ≥ 3) was identified in 28% (n = 19). Children with good outcomes based on GOS-E Peds scoring were more likely to have had normal flow velocity than other flow patterns. No other differences in neurodevelopmental outcomes were noted.
Conclusions
Individual patient responses to TBI in terms of CBFV alterations were heterogeneous. Low flow was uniformly associated with a poor outcome. Patients with good outcomes were more likely to have normal flow. This suggests CBFV may serve as a prognostic indicator in children with TBI. Future studies are needed to determine if aberrant CBFVs are also a therapeutic target.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is the major cause of morbidity and mortality in children in developed countries. Each year in the USA alone, over 475,000 children suffer head trauma [1]. The survivors of pediatric TBI and their families commonly cope with long-term motor disabilities, cognitive deficits, and behavioral issues [2,3,4]. Additionally, the economic consequences of TBI are enormous with average lifetime care costs ranging from $600,000 to $1,875,000 [5].
Following the primary injury, disturbances in cerebral circulation may play a key role in further neuronal cell injury and death and contribute to worsened neurological outcomes in these children. Arterial hypotension, focal tissue compression due to intraparenchymal hematomas, increased intracranial pressure (ICP), or vasospasm of the large intracranial vessels may result in post-traumatic cerebral ischemia [6,7,8,9,10,11,12,13,14,15]. Additionally, cerebral hyperemia following TBI is not uncommon and may result in cerebral swelling, intracranial hypertension, and secondary brain injury [10,11,12,13,14].
The cerebrovascular response of children following TBI has been evaluated using Xenon computerized tomography (XeCT) in several studies. Muizelaar et al. found that in 32 children with TBI, the average cerebral blood flow (CBF) was typically within the expected range in the first 24 h of injury [13]. Following the initial 24 h, 22% of their measurements revealed reduced CBF (< − 2 SD of normal), 49% showed normal values (− 2 SD to + 2 SD from normal), and 29% of CBF values were consistent with absolute hyperemia. They noted that children with higher flow values in the first 24 h had better neurologic outcomes, but they were unable to identify an association between later CBF velocities and outcomes after this time period. In two other studies, another author, using Xenon CT, found reduced CBF on admission across the cohorts that increased to slightly elevated values by post-injury day 2 and normalized thereafter [10, 11]. They reported uniformly poor outcomes if CBF was < 20 ml/100 g/min at any point and noted that children with overall higher CBF measurements had improved outcomes compared to those with lower CBF.
These findings suggest that CBF may have a role as a prognostic indicator and also be a therapeutic target in children following TBI. However, since these studies were published, a mandate by the Federal Drug Administration tabled the use of Xenon CT until its safety could be established. As such, surrogate markers for measuring CBF in children need to be evaluated to determine their typical pattern in the days immediately following the trauma and their associations with long-term neurodevelopmental outcomes.
Therefore, in this study, we used a transcranial Doppler (TCD) ultrasound in a cohort of children with moderate to severe TBI to describe flow velocity characteristics on days 1–8 post-injury and to examine the relationships of flow velocity to neurodevelopmental outcomes. Our hypothesis was that specific flow velocity patterns identified by transcranial Doppler ultrasound could be used to predict long-term neurologic outcomes in these children.
Materials and methods
Study population
We performed a prospective, observational study in a tertiary care, level 1 pediatric trauma center. The study was approved by the institutional review board. Informed consent was obtained from parents before enrollment. Children 1 day to 17 years of age admitted with a diagnosis of moderate to severe TBI (post-resuscitation Glasgow Coma Scale (GCS) score ≤ 12)) and abnormal brain imaging were recruited. Children were excluded if they were deemed to have suffered a non-survivable injury and had a GCS of 3 with fixed and dilated pupils. Children were also excluded if they had a previous diagnosis of severe developmental disability or mental retardation. Demographic data including age, gender, mechanism of injury, Injury Severity Score (ISS), and post-resuscitation GCS scores were obtained. Children with a post-resuscitation GCS score of 9–12 were considered to have suffered moderate TBI and those with a post-resuscitation GCS score of ≤ 8 were considered to have suffered severe TBI. Admission temperature (rectal), serum chemistry, and daily complete blood cell counts were collected prospectively. Daily pediatric intensity level of therapy (PILOT) scores were also noted.
General management protocol
All patients received routine ICU care. Patients with moderate TBI underwent frequent neurologic checks. Patients with severe TBI were treated following the Society of Critical Care Medicine Guidelines [16]. Children with mass lesions underwent primary decompressive craniotomy with resection of the mass lesion at the discretion of the neurosurgical attending physician. Other care included tracheal intubation, elevation of the head of the bed to 30°, and placement of an ICP monitor. Ventriculostomy was placed when technically feasible. Subsequent cerebrospinal fluid (CSF) drainage was taken if ventriculostomy was present. If ventriculostomy could not be inserted, an intraparenchymal monitor was placed (Camino or Licox, Integra Neurosciences, Plainsboro, NJ, USA). The type of intraparenchymal monitor that was used was left to the discretion of the neurosurgical attending physician.
All patients with severe TBI received sedation with infusions of fentanyl and Versed that were titrated according to the Faces, Legs, Activity, Cry, Consolability (FLACC) score. The FLACC score is a standardized, validated measure of pain, agitation, and sedation for children in the pediatric intensive care unit [17, 18]. The target score was < 3. Sedation infusions were increased by 10% every 10 min if FLACC scores were above the goal.
Elevation in ICP (≥ 20 mmHg) despite adequate sedation was treated with osmolar therapy followed by neuromuscular blockade. A bolus dose of hypertonic saline (5 ml/kg of 3% sodium chloride (NaCl)) was given initially. This was followed by a continuous infusion (starting at 1 ml/kg/h) to maintain sodium levels in the range of 150–165 meq/L. If ICP was ≥ 20 mmHg and serum sodium was < 150 meq/L, repeat boluses of 5 ml/kg of 3% sodium chloride were given until serum sodium level reached the goal range. For each bolus dose of hypertonic saline required, the infusion was increased by 0.5–1 ml/kg/h to maintain sodium in the target range. All patients requiring subsequent neuromuscular blockade underwent continuous electroencephalogram (EEG) monitoring.
Careful control of ventilation (PaCO2 35–45 mmHg) was undertaken in all patients. Second-tier therapies such as moderate hyperventilation (goal PaCO2 30–35 mmHg), barbiturate coma, and decompressive craniectomy were used for refractory elevations in ICP. Age-appropriate cerebral perfusion pressure (CPP) (neonates ≥ 40 mmHg, children 50–60 mmHg, adolescents > 60 mmHg) was maintained using fluid boluses to a central venous pressure > 10 cmH2O followed by an epinephrine infusion.
All patients admitted to our institution with a diagnosis of traumatic brain injury have an automatic physical medicine and rehabilitation consult at the time of admission. These patients are followed throughout their hospital course and when deemed appropriate are admitted for inpatient rehabilitation once acute inpatient needs have been completed.
Transcranial Doppler ultrasonography
Transcranial Doppler (TCD) ultrasonography was performed at the participant’s bedside by one of two sonographers using a 2-MHz pulsed probe and commercially available TCD ultrasonography unit (Sonara Digital TCD, CareFusion, Middleton, WI). The quality of the data obtained by TCD is highly influenced by operator-dependent factors such as skill and experience. Therefore, all TCD evaluations were performed by two individuals trained and experienced in TCD practices. Sonographers were tested on 10 standardized patients, and a coefficient of variation < 10% for each study measurement was demonstrated. The middle cerebral arteries (MCAs), extracranial internal carotid arteries (EC-ICAs), and basilar arteries (BAs) were insonated at 1-mm intervals using the method described by Aaslid and Lindegaard [18,19,20,21,22]. Participants underwent an initial TCD within 24 h of injury. Daily ultrasounds were continued on all study participants through hospital day 8, discharge, or death, whichever occurred first. TCD examinations were only performed if no changes to the ventilator had occurred or treatments to augment CPP had been given within the previous hour. All TCD studies were downloaded and interpreted by co-investigators blinded to the outcome of the study participant. Intra-class correlations (ICCs) were used to examine intra-rater reliability in the interpretation of TCD examinations, and adequate test-retest reliability was met with ICC ≥ 0.75.
Diagnostic criteria
Low cerebral blood flow velocity was diagnosed when measured mean CBFVs were ≤ 2 standard deviations (SD) below normal value for age and gender [23]. High cerebral blood flow velocity was diagnosed when measured mean CBFVs were ≥ 2 SDs above the normal value for age and gender. Differentiating high flow velocities from vasospasm was accomplished using the Lindegaard ratio. The ratio of flow in the MCA to EC-ICA is known as the Lindegaard ratio. In adult studies of patients with angiographically confirmed vasospasm following subarachnoid hemorrhage, values < 3 were most consistent with hyperemia as a cause of elevated flow velocities, whereas values > 3 were most consistent with vasospasm [21, 22]. No validated criteria for the diagnosis of vasospasm measured by TCD exist in children. Therefore, the adult diagnostic criteria for vasospasm following SAH were extrapolated to the current study and vasospasm was diagnosed in the anterior circulation when the mean middle cerebral artery flow velocity (Vmca) was ≥ 2 standard deviations (SD) above the age and gender normal value and the LR was ≥ 3 [15].
Clinical outcome
Participants underwent neurodevelopmental testing 12 months post-injury. All subjects completed the extended pediatric version of the Glasgow Outcome Scale (GOS-E Peds). The GOS-E Peds provides an age-appropriate, valid measurement of neurologic outcome for infants and children less than age 18 years with TBI [24]. The eight categories in the scale are (1) Upper Good Recovery, (2) Lower Good Recovery, (3) Upper Moderate Disability, (4) Lower Moderate Disability, (5) Upper Severe Disability, (6) Lower Severe Disability, (7) Vegetative State, and (8) Death. For this study, good neurologic outcome was considered to be a GOS-E Peds score ≤ 4 (lower moderate disability or better) and a poor neurologic outcome was considered to be a score of 5–8 (upper severe disability or worse). Additionally, all study participants alive in 12 months post-injury underwent testing of a measure of overall cognitive ability using age-appropriate, standardized, and validated measures (Bayley Scales of Infant Development-III for children 30 months of age or younger and age-appropriate Wechsler Intelligence Scale for children older than 30 months of age). Further testing involved appropriate tests for specific age groups. Children ages 30 months and older underwent analysis of specific cognitive abilities (verbal skills, nonverbal skills, and processing speed) using Wechsler Intelligence Scale subscales. Children age 6 and older had supplemental testing of inhibitory control (vigilance subtest of the Gordon Diagnostic System), working memory (Consonant Trigrams), and spatial planning (Tower of London). Caregivers provided ratings of adaptive functioning for all children using the Adaptive Behavior Assessment System. In children age 3 and older, parents also rated emotional and behavioral adjustment using the Strengths and Difficulties Questionnaire.
Statistics
All analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC) with two-sided p values < 0.05 considered statistically significant. Comparisons between groups were conducted using chi-squared or Fisher’s exact tests for categorical variables: t tests and one-way ANOVA (with a Satterthwaite correction for unequal group variances where necessary) or Wilcoxon rank sum tests and Kruskal-Wallis tests for continuous variables.
Results
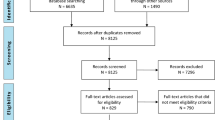
Eighty-three patients were screened. Six suffered injuries deemed non-survivable and were excluded. An additional two patients were excluded due to previously diagnosed developmental disability. In four patients, no parent was available to give consent and another two guardians refused to consent for the study. Sixty-nine patients were enrolled and underwent daily TCD of cerebral blood flow velocities. Demographic, clinical, and radiographic characteristics of participants are summarized in Table 1. The cohort consisted of 35 children with moderate TBI (defined as post-resuscitation GCS of 9–12). Median GCS in this group was 10 (range 9–12). Thirty-four children suffered severe TBI (defined as post-resuscitation GCS ≤ 8) with a median GCS in these children of 5 (range 3–8). There were no significant differences in hematocrit, mean arterial pressure, or PaCO2 between groups that could account for alterations in measured flow velocities reported below (data not shown). All 69 patients completed the GOS-E Peds at 12 months post-injury. Four patients died, five were in a persistent vegetative state, and three were severely disabled 12 months from trauma so they could not complete further neurodevelopmental testing beyond the GOS-E Peds. Of the remaining 57, eight children failed to return for neurodevelopmental testing leaving 49 participants that underwent testing outlined above.
Low flow velocities (< 2 SD below age and gender normal) were found in 6% (n = 4) of patients. Their day-to-day flow velocities are shown in Fig. 1. Three of these patients died, and one remained in a persistent vegetative state 1 year after injury. Due to the relatively few patients with low flow velocities, clinical characteristics of patients with this pattern on TCD examination could not be evaluated. Because of the poor outcomes of these children, none completed neurodevelopmental testing. Notably, no patient with a good neurologic outcome (GOS-E Peds ≤ 4) had a single flow velocity measurement in the low flow.
Daily flow velocity measurements in children meeting criteria for low cerebral blood flow velocity. Three children had progressive decreased flow velocities despite maximal medical and surgical management of their traumatic brain injury and died. One child had initially decreased flow velocities that improved but still remained below the normal range throughout the study period. This child remained in a persistent vegetative state
In the remaining children, normal flow velocities (± 2 SD around age and gender normal) were seen in 43% (n = 30). Day-to-day flow velocity measurements for these participants are shown in Fig. 2. High flow velocities (> 2 SD above age and gender normal with LR < 3) were identified in 23% of children (n = 16), and high flow velocities with high LR that we classified as vasospasm (> 2SD above age and gender normal with LR ≥ 3) were identified in 28% (n = 19) (Fig. 3).
Children who developed high flow velocities with high LR (“vasospasm” according to our criteria) were more likely than those with high or normal flow to have been involved in a motor vehicle accident (47 vs 6% (high flow velocity with low LR) and 20% (normal flow velocity), p = 0.03), have lower GCS scores (7 (IQR 6,9) vs 8.5 (IQR 6.5,10) (high flow velocity) and 9 (8,10) (normal flow velocity), p = 0.01), experience hyperthermia in the intensive care unit (68 vs 44% (high flow velocity) and 30% (normal flow velocity), p = 0.03), have higher average PILOT scores (4.2 (IQR 2,9.25) vs 3.6 (IQR 2.33,6) (high flow velocity) vs 1.5 (IQE 1,3) (normal flow velocity), p = 0.006), and have longer ICU stays (10 days (IQR 7,14) vs 3 (IQR 1,5.5) (high flow velocity) vs 1 (IQR 1,3) (normal flow velocity), p ≤ 0.001) (Tables 2 and 3). Children with normal or high flow without vasospasm were not significantly more likely to have any specific clinical characteristics than other types of flow velocities (Tables 2 and 3).
Of the 69 patients involved in the study, 30 were admitted to acute inpatient rehabilitation. Patients were not admitted to rehabilitation if they were under age 2 (n = 16), functioning at too high or low a level to benefit from inpatient rehabilitation (low functional status, n = 5, high functional status, n = 14), or if they died (n = 4) as a result of their injuries. The mean length of stay on rehabilitation was 29 days. Patients were discharged once they had neared a functional plateau and when they were at a level of function that would allow for safe home discharge.
In children who suffered moderate TBI (GCS 9–12), 54% had normal flow velocities and a good neurologic outcome (GOS-E Peds ≤ 4) and 6% had normal flow velocities and a poor neurologic outcome (Fig. 4). In this group, an additional 16% had high flow velocities and a good neurologic outcome and 6% had high CBFV and a poor neurologic outcome (Fig. 4). Twelve percent of children with moderate TBI and vasospasm as we defined it had good neurologic outcomes, and 6% had vasospasm with poor neurologic outcomes (Fig. 4).
In children with severe TBI (GCS ≤ 8), four had low flow velocities as noted above (3 died and 1 had a vegetative outcome) (Fig. 5). Additionally, 18% had normal flow velocities and good neurologic outcomes and 9% had normal flow velocities with poor neurologic outcomes (Fig. 5). Twelve percent of these children with severe TBI and high flow velocities had good outcomes, 9% had a severe/vegetative outcome, and 3% died (Fig. 5). Patients with severe TBI and CBFV meeting our criteria for vasospasm had a good neurologic outcome 18% of the time and a poor outcome 21% of the time (Fig. 5).
The three groups of children (normal CBFV, high CBFV, vasospasm) showed no significant differences in overall cognitive ability, verbal skills, nonverbal skills, processing speed, inhibitory control, working memory, spatial planning, adaptive functioning, or emotional and behavioral adjustment (data not shown).
Discussion
Aberrant CBF may serve as a prognostic indicator of neurologic outcomes in children following TBI. Abnormal CBF may also be an important therapeutic target for reducing secondary brain injury in this cohort. Previous clinical studies have used xenon CT scans to characterize CBF, but this modality is no longer available. Therefore, the primary purpose of this investigation was to characterize flow velocity patterns on TCD following moderate to severe TBI and to determine if specific changes in these flow patterns were associated with neurodevelopmental outcomes. Our initial cohort involved 69 children, all of whom underwent daily TCD evaluation of CBFVs and had GOS-E Peds scores obtained at 12 months after injury. Additionally, 49 children underwent neurodevelopmental testing to evaluate cognitive ability, adaptive functioning, and emotional/behavioral adjustment 1 year post-injury.
We had several key findings. Across our entire cohort, normal flow velocities were seen in 43% of children, high flow velocities in 23%, high flow velocities meeting our criteria for vasospasm in 28%, and low flow velocities in 6%. These findings are consistent with another study that identified a heterogeneous response of CBF to TBI in children [13]. In this other study, 49% of participants were noted to have normal flow, 29% experienced hyperemia, and 22% had low flow at variable time points from injury. In another study involving 21 children, cerebral blood flow values varied considerably within and between patients from day to day following admission for TBI [14]. These findings suggest that no uniform cerebrovascular response to TBI is seen in children. Identifying day to day changes in children’s CBFV after TBI may provide important insight into specific changes occurring in their unique physiology.
Another key finding is that any child who experienced a single low flow velocity (< 2 SD below age and gender normal) had a poor neurologic outcome (death or vegetative state). Conversely, patients with consistently normal flow velocities did not die and were more likely to have good outcomes than those children with abnormal flow velocities. Early hypoperfusion and the relationship with poor outcome after TBI observed in our cohort mirrors the results of other studies (10–11). Specifically, in these other studies, poor outcome was seen uniformly if CBF was < 20 ml/100 g/min. Additionally, children with favorable outcomes were more likely to have higher CBF (61 ml/100 g/min in those with good outcome compared to 46 ml/100 g/min in those with poor outcome). These findings suggest that low CBFV likely represents a prognostic variable that may aid in clinical decision-making and in counseling families. Further studies are needed to determine if it also represents a therapeutic target that, if successfully treated, would improve outcomes in children with traumatic brain injury. Potential interventions that could be explored in children with low cerebral flow include mechanical ventilator adjustments to allow arterial CO2 to rise and subsequently cerebral blood flow to increase if intracranial pressure (ICP) is adequately controlled. Another possible option to explore would be if low cerebral flow can be improved with more aggressive ICP management regardless of the absolute ICP value (i.e., even if ICP is at or below the traditional goal of 20, would further treatments and reductions of this ICP to < 10–15 help normalize cerebral flow).
In a previous report, we found worse neurologic outcomes 1 month from the time of injury (by GOS-E Peds scoring) in children who met our diagnostic criteria for vasospasm than in those children who did not [15]. Interestingly, when looking at long-term neurologic outcomes 1 year from the time of injury, children falling into this category of flow velocity seem to do as well as children in other categories (excluding those children with low CBFV). On further review of these 19 children with vasospasm as we defined it, 16 of them underwent acute inpatient rehabilitation (3 infants were excluded based on their age being too young to benefit). Therefore, while they made up only 28% of our entire cohort, children with vasospasm made up more than 50% of the cases undergoing inpatient rehabilitation. While there are a number of confounders (lower admission GCS scores, higher PILOT scores in children with vasospasm) that make definitive conclusions difficult to make, an interesting consideration is that vasospasm in the ICU may predict a lower level of functionality in the acute and subacute phase. Even more importantly would be the consideration that despite this, with aggressive rehabilitation, these children have the potential to do equally well long-term from a neurodevelopmental standpoint. Vasospasm too, if identified and confirmed, may serve as a therapeutic target to decrease secondary brain injury in some children. If ICP is relatively well controlled, traditional triple-H therapy (hypertensive, hypervolemic, hemodilution) or portions of triple H therapy may be one option. If ICP is poorly controlled or a suspicion that autoregulation is not intact is present, another therapeutic option for vasospasm may be direct or systemic calcium channel blockers [25].
A number of additional important limitations of the study need to be considered. TCD is an indirect measure of cerebral blood flow, and the correlation of TCD flow velocities with absolute cerebral flow remains uncertain in pediatric patients. One recent study in healthy adult patients found a strong positive correlation between regional cerebral blood flow on perfusion MRI when compared to TCD flow velocities, thus validating Doppler measurements of CBF velocity as indicative of CBF in adults [26]. Further studies similar to this are needed in children and in those who have experienced cerebral insult to determine the relationship between absolute cerebral blood flow and TCD flow velocities in these populations. Furthermore, in our study, concurrent measures of metabolic demand were not obtained. Therefore, marked elevations in CBFV cannot truly be called hyperemia and, similarly, marked reductions do not necessarily prove ischemia has occurred because definitive conclusions depend on the metabolic state of the child at the time the TCD is performed. Likewise, another important limitation to note is that TCD criteria for vasospasm have never been validated in children with traumatic brain injury. We extrapolated our TCD diagnostic criteria for vasospasm from studies in adult patients who had angiographically confirmed vasospasm following subarachnoid hemorrhage. Definitive confirmation of vasospasm with angiography once meeting our diagnostic criteria by TCD was not performed. However, six of the 19 children had clinically indicated magnetic resonance arteriography (MRA) scans performed during the time vasospasm criteria by TCD was met and all had a documented decrease in vessel diameter between 50 and 75%. Two other children had noted neurologic declines with GCS scores falling from 12 to 8 in one child and from 8 to 5 in another child hours after vasospasm was diagnosed based on our criteria. There were no other imaging findings to suggest alternative causes to their neurologic decline. Future studies should work to validate these TCD criteria for vasospasm with angiography or, potentially, MRA before widespread use of these criteria is adopted. Furthermore, until TCD criteria for vasospasm are validated, interventions for a child with TCD diagnosed vasospasm are not indicated until the vasospasm is confirmed with a secondary test and until the treating team determines the degree of vasospasm is clinically relevant.
Lastly, the cohort is relatively small, as already mentioned, and only a small number of participants were identified with each type of flow velocity pattern. This specifically is important to note in the low cerebral flow group given that there were only four children that fell into this category. In order to generalize the statement that children with low flow measured by TCD have poor outcomes, further larger studies with more patients meeting these criteria need to be performed. Also, because of the wide age range of subjects enrolled in the study, only a limited number of neurodevelopmental outcome measures are applicable for all participants. This makes comparison across the group as a whole problematic and may have contributed to our non-significant results on neurodevelopmental testing when attempting to compare different flow velocity groups. Future studies with larger numbers of children in each age group with various flow velocity patterns may yield more robust information regarding the impact of CBF changes on important neurodevelopmental outcomes such as cognitive ability, verbal and nonverbal skills, processing speed, inhibitory control, working memory, spatial planning, adaptive functioning, and emotional/behavioral adjustment. Additionally, we did not correct for multiple testing in our cohort as we did not find statistically significant differences in neurodevelopmental outcomes. However, future studies, if differences are identified, should correct p values for multiple testing given the day-to-day variability in TCD flow velocity values and the number of neurodevelopmental tests that are carried out.
Conclusions
CBFV alterations in individual children following TBI were heterogenous. Low flow was uniformly associated with a poor neurologic outcome. Patients with good outcomes were more likely to have normal flow. These results suggest CBFVs measured using TCD may serve as a useful prognostic indicator in children with TBI. Further studies are needed to determine if these alterations may also serve as a therapeutic target to improve neurologic outcomes in children with TBI.
References
Faul M, Xu L, Wald MM et al (2010) Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta, GA, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control
Schwartz L, Taylor HG, Drotar D, Yeates KO, Wade SL, Stancin T (2003) Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. J Pediatr Psychol 28(4):251–263. https://doi.org/10.1093/jpepsy/jsg013
Ewing-Cobbs L, Miner ME, Fletcher JM, Levin HS (1989) Intellectual, motor, and language sequelae following closed head injury in infants and preschoolers. J Pediatr Psychol 14(4):531–547. https://doi.org/10.1093/jpepsy/14.4.531
Levin HS, Eisenberg HM, Wigg NR, Kobayashi K (1982) Memory and intellectual ability after head injury in children and adolescents. Neurosurgery 11(5):668–673. https://doi.org/10.1227/00006123-198211000-00009
Finkelstein E, Corso P, Miller T (2006) The incidence and economic burden of injuries in the United States. Oxford University Press, New York. https://doi.org/10.1093/acprof:oso/9780195179484.001.0001
Graham DI, Adams JH, Doyle D (1978) Ischaemic brain damage in fatal non-missile head injuries. J Neurol Sci 39(2-3):213–234. https://doi.org/10.1016/0022-510X(78)90124-7
Catala-Temprano A, Claret Teruel G, Cambra Lasaosa FJ et al (2007) Intracranial pressure and cerebral perfusion pressure as risk factors in children with traumatic brain injuries. J Neurosurg 106(6 Suppl):463–466. https://doi.org/10.3171/ped.2007.106.6.463
Allen BB, Chiu YL, Gerber LM, Ghajar J, Greenfield JP (2014) Age-specific cerebral perfusion pressure thresholds and survival in children and adolescents with severe traumatic brain injury. Pediatr Crit Care Med 15(1):62–70. https://doi.org/10.1097/PCC.0b013e3182a556ea
Ferguson N, Shein SL, Kochanek PM et al (2016) Intracranial hypertension and cerebral hypoperfusion in children with severe traumatic brain injury: thresholds and burden in accidental and abusive insults. Pediatr Crit Care Med 17(5):444–450. https://doi.org/10.1097/PCC.0000000000000709
Adelson PD, Clyde B, Kochanek PM, Wisniewski SR, Marion DW, Yonas H (1997) Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr Neurosurg 26(4):200–207. https://doi.org/10.1159/000121192
Adelson PD, Srinivas R, Chang Y, Bell M, Kochanek PM (2011) Cerebrovascular response in children following severe traumatic brain injury. Childs Nerv Syst 27(9):1465–1476. https://doi.org/10.1007/s00381-011-1476-z
Bruce DA, Alavi A, Bilaniuk LT, Dolinskas C, Obrist W, Uzzell B (1981) Diffuse cerebral swelling following head injuries in children: the syndrome of ‘malignant brain edema’. J Neurosurg 54(2):170–178. https://doi.org/10.3171/jns.1981.54.2.0170
Muizelaar JP, Marmarou A, DeSalles AF et al (1989) Cerebral blood flow and metabolism in severely head-injured children. J Neurosurg 71(1):63–71. https://doi.org/10.3171/jns.1989.71.1.0063
Sharples PM, Stuart AG, Matthews DSF, Aynsley-Green A, Eyre JA (1995) Cerebral blood flow and metabolism in children with severe head injury. Part 1: relation to age, Glasgow coma score, outcome, intracranial pressure, and time after injury. J Neurol Neurosurg Psychiatry 58(2):145–152. https://doi.org/10.1136/jnnp.58.2.145
O’Brien NF, Maa T, Yeates KO (2015) The epidemiology of vasospasm in children with moderate to severe traumatic brain injury. Crit Care Med 43(3):674–685. https://doi.org/10.1097/CCM.0000000000000745
Kochanek PM, Carney N, Adelson PD, American Academy of Pediatrics-Section on Neurological Surgery; American Association of Neurological Surgeons/Congress of Neurological Surgeons; Child Neurology Society; European Society of Pediatric and Neonatal Intensive care; Neurocritical Care Society; Pediatric Neurocritical Care Research Group; Society of Critical Care medicine; Pediatric Intensive Care Society UK; Society for Neuroscience in Anesthesiology and Critical Care; World Federation of Pediatric Intensive and Critical Care Societies et al (2012) Guidelines for the acute management of severe traumatic brain injury in infants, children, and adolescents-second edition. Pediatr Crit Care Med 13(Suppl 1):S1–82
Merkel S, Voepel-Lewis T, Shayevitz J, Malviya S (1997) The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs 23(3):293–297
Voepe-Lewis T, Zanotti J, Dammeyer JA, Merkel S (2010) Reliability and validity of the face, legs, activity, cry, consolability behavioral tool in assessing acute pain in critically ill patients. Am J Crit Care 19(1):55–61. https://doi.org/10.4037/ajcc2010624
Aaslid R, Markwalder TM, Nornes H (1982) Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 57(6):769–774. https://doi.org/10.3171/jns.1982.57.6.0769
Aaslid R, Huber P, Nornes H (1984) Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg 60(1):37–41. https://doi.org/10.3171/jns.1984.60.1.0037
Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P (1989) Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir 100(1-2):12–24. https://doi.org/10.1007/BF01405268
Bode H, Wais U (1988) Age dependence of flow velocities in basal cerebral arteries. Arch Dis Child 63(6):606–611. https://doi.org/10.1136/adc.63.6.606
O’Brien NF (2015) Reference values for cerebral blood flow velocities in critically ill, sedated children. Childs Nerv Syst 31(12):2269–2276. https://doi.org/10.1007/s00381-015-2873-5
Beers SR, Wisniewski SR, Garcia-Fillion P et al (2012) Validity of a pediatric version of the Glasgow outcome scale-extended. J Neurotrauma 29(6):1126–1139. https://doi.org/10.1089/neu.2011.2272
Armin SS, Colohan AR, Zhang JH (2006) Traumatic subarachnoid haemorrhage: our current understanding and its evolution over the past half century. Neurol Res 28(4):445–452. https://doi.org/10.1179/016164106X115053
Sorond FA, Hollenberg NK, Panych LP, Fisher NDL (2010) Brain blood flow and velocity, correlations between magnetic resonance imaging and transcranial Doppler sonography. J Ultrasound Med 29(7):1017–1022. https://doi.org/10.7863/jum.2010.29.7.1017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
O’Brien, N.F., Maa, T., Moore-Clingenpeel, M. et al. Relationships between cerebral flow velocities and neurodevelopmental outcomes in children with moderate to severe traumatic brain injury. Childs Nerv Syst 34, 663–672 (2018). https://doi.org/10.1007/s00381-017-3693-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3693-6