Abstract
Background
Germ cell tumors (GCT) are the most common central nervous system (CNS) tumors in individuals with Down syndrome. Patients with Down syndrome treated with CNS irradiation are at increased risk of developing cerebrovascular complications such as moyamoya disease. Embryonal carcinoma components are recognized to be more resistant to conventional chemotherapy and radiotherapy and confer a very poor prognosis. CD30 is a member of the tumor necrosis factor-receptor superfamily. CD30+ has a limited expression in normal cells but is the defining marker for embryonal carcinoma. Brentuximab-vedotin is a novel antibody-drug conjugate consisting of the chimeric anti-CD30 antibody conjugated to an anti-tubulin synthetic analog monomethyl auristatin E.
Methods
A retrospective review of the patient’s records was conducted in September 2017.
Results
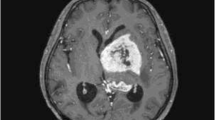
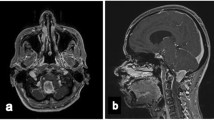
We report upon our management of a teenage girl with Down syndrome and a suprasellar pure embryonal carcinoma utilizing an intensive chemotherapy regimen followed by brentuximab-vedotin without irradiation. The patient received two cycles of carboplatin and etoposide interspersed with one cycle of cyclophosphamide and etoposide for induction followed by three cycles of marrow-ablative thiotepa and carboplatin rescued by autologous hematopoietic stem cell. Finally, She received six cycles of intravenous brentuximab-vedotin. The patient continues without evidence of recurrent tumor by MRI and tumor marker surveillance 24 months since diagnosis, with no adverse sequelae of her treatment.
Conclusions
Brentuximab-vedotin may provide a selective and safe alternative (or adjunct) to radiotherapy in the management of patients with CD30-positive CNS embryonal carcinoma, especially for those patients at high risk of developing irradiation-related complications.


Similar content being viewed by others
References
Sugimoto K, Ideguchi M, Sadahiro H, Yoshikawa K, Goto H, Nomura S, Fujii M, Suzuki M (2013) Yolk sac tumor of the bilateral basal ganglia in a patient with Down syndrome. Brain Tumor Pathol 30(4):247–252. https://doi.org/10.1007/s10014-012-0134-9
Satgé D, Monteil P, Sasco AJ, Vital A, Ohgaki H, Geneix A, Malet P, Vekemans M, Réthoré MO (2001) Aspects of intracranial and spinal tumors in patients with Down syndrome and report of a rapidly progressing grade 2 astrocytoma. Cancer 91(8):1458–1466. https://doi.org/10.1002/1097-0142(20010415)91:8<1458::AID-CNCR1153>3.0.CO;2-B
Junqueira PA and Moura-Ribeiro MVLd (2002) Moyamoya and Down syndrome: study conducted by meta-analysis. Arq Neuropsiquiatr 2002;60(2A):274–280, DOI: https://doi.org/10.1590/S0004-282X2002000200017
de Borchgrave V, Saussu F, Depre A, de Barsy T (2002) Moyamoya disease and Down syndrome: case report and review of the literature. Acta Neurol Belg 102(2):63–66
Dai AI, Shaikh ZA, Cohen ME (2000) Early-onset moyamoya syndrome in a patient with Down syndrome: case report and review of the literature. J Child Neurol 15(10):696–699. https://doi.org/10.1177/088307380001501012
Fung C, Kwong K, Tsui E, Wong S (2003) Moyamoya syndrome in a child with Down syndrome. Hong Kong Med J 9(1):63–66
Phi JH, Wang K-C, Lee JY, Kim S-K (2015) Moyamoya syndrome: a window of moyamoya disease. J Korean Neurosurg Soc 57(6):408–414. https://doi.org/10.3340/jkns.2015.57.6.408
Calaminus G, Frappaz D, Kortmann RD, Krefeld B, Saran F, Pietsch T, Vasiljevic A, Garre ML, Ricardi U, Mann JR, Göbel U, Alapetite C, Murray MJ, Nicholson JC (2017) Outcome of patients with intracranial non-germinomatous germ cell tumors-lessons from the SIOP-CNS-GCT-96 trial. Neuro-Oncology 19(12):1661–1672. https://doi.org/10.1093/neuonc/nox122
Matsutani M (2004) Clinical management of primary central nervous system germ cell tumors. Semin Oncol 31(5):676–683. https://doi.org/10.1053/j.seminoncol.2004.07.010
Berger GK, Gee K, Votruba C, McBride A, Anwer F (2017) Potential application and prevalence of the CD30 (Ki-1) antigen among solid tumors: a focus review of the literature. Crit Rev Oncol Hematol 113:8–17. https://doi.org/10.1016/j.critrevonc.2017.02.021
Deutsch YE, Tadmor T, Podack ER, Rosenblatt JD (2011) CD30: an important new target in hematologic malignancies. Leuk Lymphoma 52(9):1641–1654. https://doi.org/10.3109/10428194.2011.574761
Squillante CM, Vaughn DJ (2015) Targeted therapies in germ cell tumors. Urol Oncol 33(8):363–369. https://doi.org/10.1016/j.urolonc.2014.09.008
Giannatempo P, Paolini B, Miceli R, Raggi D, Nicolai N, Farè E, Catanzaro M, Biasoni D, Torelli T, Stagni S, Piva L, Mariani L, Salvioni R, Colecchia M, Gianni AM, Necchi A (2013) Persistent CD30 expression by embryonal carcinoma in the treatment time course: prognostic significance of a worthwhile target for personalized treatment. J Urol 190(5):1919–1924. https://doi.org/10.1016/j.juro.2013.04.057
Ansell SM (2014) Brentuximab vedotin. Blood 124(22):3197–3200. https://doi.org/10.1182/blood-2014-06-537514
Albany C, Feldman DR, Garbo LE and Einhorn LH (2013) Antitumor activity of brentuximab vedotin in CD30 positive refractory germ cell tumors. J Clin Oncol 31:6_suppl, 327–327
Necchi A, Anichini A, Raggi D, Giannatempo P, Magazzù D, Nicolai N, Colecchia M, Paolini B, Coradeschi E, Tassi E, Grazia G, Mortarini R, Calareso G, De Fato R, Togliardi E, Crippa F, Salvioni R, Valagussa P, Gianni AM (2016) Brentuximab vedotin in CD30-expressing germ cell tumors after chemotherapy failure. Clin Genitourin Cancer 14(4):261–264. https://doi.org/10.1016/j.clgc.2016.03.020
Abid MB, Wang S, Loi HY, Poon L (2016) ALK-negative anaplastic large cell lymphoma with CNS involvement needs more than just brentuximab vedotin. Ann Hematol 95(10):1725–1726. https://doi.org/10.1007/s00277-016-2746-3
Carson KR1, Newsome SD, Kim EJ, Wagner-Johnston ND, von Geldern G, Moskowitz CH, Moskowitz AJ, Rook AH, Jalan P, Loren AW, Landsburg D, Coyne T, Tsai D, Raisch DW, Norris LB, Bookstaver PB, Sartor O and Bennett CL (2014) Progressive multifocal leukoencephalopathy associated with brentuximab vedotin therapy: a report of 5 cases from the Southern Network on Adverse Reactions (SONAR) project. Cancer 120(16):2464–2471
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Abu Arja, M.H., Conley, S.E., Salceda, V. et al. Brentuximab-vedotin maintenance following chemotherapy without irradiation for primary intracranial embryonal carcinoma in down syndrome. Childs Nerv Syst 34, 777–780 (2018). https://doi.org/10.1007/s00381-017-3690-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3690-9