Abstract
Purpose
The purpose of this study was to examine age-related, infratentorial changes in T2 relaxation times during infancy and childhood using routine MRI data at 3 Tesla.
Methods
One hundred patients (0–199 months) without signal abnormalities on conventional MRI were retrospectively selected from our pool of pediatric MRI examinations. T2 maps based on our routinely acquired triple-echo turbo spin-echo (TSE) sequence were created. Based on their clinical symptoms, the children were divided into 43 controls and 57 diseased children with different clinical diseases. T2 relaxation times were measured in 15 infratentorial brain regions (medullary pyramid, ventral and dorsal pons, middle cerebellar peduncle, dentate nucleus, medial and lateral cerebellar hemisphere each on both sides, and in the cerebellar vermis) investigating age-related changes. Secondly, this study examined whether those changes in T2 values differed between healthy and diseased children.
Results
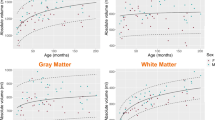
Age significantly reduced T2 relaxation time in all infratentorial brain regions (p < 0.05). With increasing age, the T2 relaxation times decreased continuously, faster in the first 9 months and slower thereafter. Overall, controls did not differ significantly from diseased children (p > 0.05) apart from the dentate nucleus and cerebellar hemispheres in terms of rapid decline (larger in controls) and the right dorsal pons and left pyramid in terms of slow decline (larger in diseased children). In both groups, the later slow decline was almost negligible.
Conclusions
Using T2 maps, it was possible to determine age-related T2 relaxation times in the different infratentorial brain regions in this preliminary study. Between neurologically healthy controls and diseased children, no significant differences in T2 relaxation times could be found overall in the studied regions.




Similar content being viewed by others
References
Autti T, Raininko R, Vanhanen SL, Kallio M, Santavuori P (1994) MRI of the normal brain from early childhood to middle age. II. Age dependence of signal intensity changes on T2-weighted images. Neuroradiology 36:649–651
Baierl P, Forster C, Fendel H, Naegele M, Fink U, Kenn W (1988) Magnetic resonance imaging of normal and pathological white matter maturation. Pediatr Radiol 18:183–189
Barkovich AJ (2000) Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol 21:1099–1109
Barkovich AJ, Kjos BO, Jackson DE Jr, Norman D (1988) Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology 166:173–180. doi:10.1148/radiology.166.1.3336675
Barkovich AJ, Maroldo TV (1993) Magnetic resonance imaging of normal and abnormal brain development. Top Magn Reson Imaging: TMRI 5:96–122
Bojorquez JZ, Bricq S, Acquitter C, Brunotte F, Walker PM, Lalande A (2017) What are normal relaxation times of tissues at 3 T? Magn Reson Imaging 35:69–80. doi:10.1016/j.mri.2016.08.021
Brooks DJ, Luthert P, Gadian D, Marsden CD (1989) Does signal-attenuation on high-field T2-weighted MRI of the brain reflect regional cerebral iron deposition? Observations on the relationship between regional cerebral water proton T2 values and iron levels. J Neurol Neurosurg Psychiatry 52:108–111
Christophe C, Muller MF, Baleriaux D, Kahn A, Pardou A, Perlmutter N, Szliwowski H, Segebarth C (1990) Mapping of normal brain maturation in infants on phase-sensitive inversion-recovery MR images. Neuroradiology 32:173–178
Ding XQ, Kucinski T, Wittkugel O, Goebell E, Grzyska U, Gorg M, Kohlschutter A, Zeumer H (2004) Normal brain maturation characterized with age-related T2 relaxation times: an attempt to develop a quantitative imaging measure for clinical use. Investig Radiol 39:740–746
Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Huppi PS, Hertz-Pannier L (2014) The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 276:48–71. doi:10.1016/j.neuroscience.2013.12.044
Engelbrecht V, Rassek M, Preiss S, Wald C, Modder U (1998) Age-dependent changes in magnetization transfer contrast of white matter in the pediatric brain. AJNR Am J Neuroradiol 19:1923–1929
Ferrie JC, Barantin L, Saliba E, Akoka S, Tranquart F, Sirinelli D, Pourcelot L (1999) MR assessment of the brain maturation during the perinatal period: quantitative T2 MR study in premature newborns. Magn Reson Imaging 17:1275–1288
Holland BA, Haas DK, Norman D, Brant-Zawadzki M, Newton TH (1986) MRI of normal brain maturation. AJNR Am J Neuroradiol 7:201–208
Lu H, Nagae-Poetscher LM, Golay X, Lin D, Pomper M, van Zijl PC (2005) Routine clinical brain MRI sequences for use at 3.0 Tesla. J Magn Reson Imaging: JMRI 22:13–22. doi:10.1002/jmri.20356
McArdle CB, Richardson CJ, Nicholas DA, Mirfakhraee M, Hayden CK, Amparo EG (1987) Developmental features of the neonatal brain: MR imaging. Part I. Gray-white matter differentiation and myelination. Radiology 162:223–229. doi:10.1148/radiology.162.1.3786767
Montgomery D, Peck E, Vining G (2012) Introduction to linear regression analysis, 5th edn. Wiley, Hoboken
Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, McKinstry RC (2001) Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology 221:349–358. doi:10.1148/radiol.2212001702
Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A (2001) Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull 54:255–266
Rademacher J, Engelbrecht V, Burgel U, Freund H, Zilles K (1999) Measuring in vivo myelination of human white matter fiber tracts with magnetization transfer MR. NeuroImage 9:393–406
Sakuma H, Nomura Y, Takeda K, Tagami T, Nakagawa T, Tamagawa Y, Ishii Y, Tsukamoto T (1991) Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion-weighted MR imaging. Radiology 180:229–233. doi:10.1148/radiology.180.1.2052700
Sarikaya B, McKinney AM, Spilseth B, Truwit CL (2013) Comparison of spin-echo T1- and T2-weighted and gradient-echo T1-weighted images at 3T in evaluating very preterm neonates at term-equivalent age. AJNR Am J Neuroradiol 34:1098–1103. doi:10.3174/ajnr.A3323
Schmitz BL, Gron G, Brausewetter F, Hoffmann MH, Aschoff AJ (2005) Enhancing gray-to-white matter contrast in 3T T1 spin-echo brain scans by optimizing flip angle. AJNR Am J Neuroradiol 26:2000–2004
Staudt M, Krageloh-Mann I, Grodd W (2000) Normal myelination in childhood brains using MRI--a meta analysis. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 172:802–811. doi:10.1055/s-2000-7898
Telford EJ, Cox SR, Fletcher-Watson S, Anblagan D, Sparrow S, Pataky R, Quigley A, Semple SI, Bastin ME, Boardman JP (2017) A latent measure explains substantial variance in white matter microstructure across the newborn human brain. Brain Struct Funct. doi:10.1007/s00429-017-1455-6
Thornton JS, Amess PN, Penrice J, Chong WK, Wyatt JS, Ordidge RJ (1999) Cerebral tissue water spin-spin relaxation times in human neonates at 2.4 tesla: methodology and the effects of maturation. Magn Reson Imaging 17:1289–1295
Tyan AE, McKinney AM, Hanson TJ, Truwit CL (2015) Comparison of spin-echo and gradient-echo T1-weighted and spin-echo T2-weighted images at 3T in evaluating term-neonatal myelination. AJNR Am J Neuroradiol 36:411–416. doi:10.3174/ajnr.A4099
van der Knaap MS, Valk J (2005) Magnetic resonance of myelination and myelin disorders, 3rd edn. Springer, Berlin
van der Knaap MS, Valk J (1990) MR imaging of the various stages of normal myelination during the first year of life. Neuroradiology 31:459–470
Yakovlev P, Lecours A (1967) The myelogenetic cycles of regional maturation of the brain. In: Minkowski A (ed) Regional development of the brain in early life. Blackwell Scientific, Oxford, pp 3–70
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest in relation to this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Funding
No funding was received.
Rights and permissions
About this article
Cite this article
Bültmann, E., Spineli, L.M., Göhner, F. et al. Age-related T2 relaxation times at 3 Tesla as a biomarker of infratentorial brain maturation. Childs Nerv Syst 34, 117–127 (2018). https://doi.org/10.1007/s00381-017-3561-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3561-4