Abstract
Purpose
Amplification and high levels of NOTCH ligand expression have been identified in several types of pediatric brain tumors. A phase I trial of weekly MK-0752, an oral inhibitor of gamma-secretase, was conducted in children with recurrent central nervous system (CNS) malignancies to estimate the maximum tolerated dose, dose-limiting toxicities (DLT), pharmacokinetics (PK), and pharmacodynamics of weekly MK-0752.
Methods
MK-0752 was administered once weekly at 1000 and 1400 mg/m2 using a rolling-6 design. PK analysis was performed during the first course. NOTCH and HES expression was assessed by immunohistochemistry and Western blot.
Results
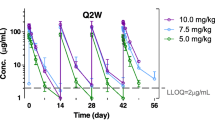
Ten eligible patients were enrolled (median age 8.8 years; range 3.1–19.2) with diagnoses of brain stem glioma (n = 3), ependymoma (n = 2), anaplastic astrocytoma (n = 1), choroid plexus carcinoma (n = 2), medulloblastoma (n = 1), and primitive neuroectodermal tumor (n = 1). Nine were evaluable for toxicity. One DLT of fatigue occurred in the six evaluable patients enrolled at 1000 mg/m2/dose. No DLTs were experienced by three patients treated at 1400 mg/m2/dose. Non-dose-limiting grade 3 toxicities included lymphopenia, neutropenia, and anemia. Median number of treatment courses was 2 (range 1–10). Two patients continued on therapy for at least 6 months. The median (range) Cmax of MK-0752 was 88.2 μg/mL (40.6 to 109 μg/mL) and 60.3 μg/mL (59.2 to 91.9 μg/mL) in patients receiving 1000 and 1400 mg/m2/week, respectively. NOTCH expression was decreased in six of seven patients for whom tissue was available at 24 h post-MK-0752.
Conclusion
MK-0752 is well tolerated and exhibits target inhibition at 1000 and 1400 mg/m2/week in children with recurrent CNS malignancies.


Similar content being viewed by others
References
Miele L (2006) Notch signaling. Clin Cancer Res 12:1074–1079. doi:10.1158/1078-0432.CCR-05-2570
Takebe N, Harris PJ, Warren RQ, Ivy SP (2011) Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol 8:97–106. doi:10.1038/nrclinonc.2010.196
Lu P, Bai X-C, Ma D et al (2014) Three-dimensional structure of human γ-secretase. Nature 512:166–170. doi:10.1038/nature13567
Tonon G, Modi S, Wu L et al (2003) t (11;19) (q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet 33:208–213. doi:10.1038/ng1083
Chen Y, McGee J, Chen X et al (2014) Identification of druggable cancer driver genes amplified across TCGA datasets. PLoS One 9:e98293. doi:10.1371/journal.pone.0098293
Fan X, Mikolaenko I, Elhassan I et al (2004) Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res 64:7787–7793. doi:10.1158/0008-5472.CAN-04-1446
Hatakeyama J, Sakamoto S, Kageyama R (2006) Hes1 and Hes5 regulate the development of the cranial and spinal nerve systems. Dev Neurosci 28:92–101. doi:10.1159/000090756
Li Y, Hibbs MA, Gard AL et al (2012) Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells 30:741–752. doi:10.1002/stem.1030
Cuevas IC, Slocum AL, Jun P et al (2005) Meningioma transcript profiles reveal deregulated Notch signaling pathway. Cancer Res 65:5070–5075. doi:10.1158/0008-5472.CAN-05-0240
Beschorner R, Waidelich J, Trautmann K et al (2013) Notch receptors in human choroid plexus tumors. Histol Histopathol 28:1055–1063
Fiaschetti G, Schroeder C, Castelletti D et al (2014) NOTCH ligands JAG1 and JAG2 as critical pro-survival factors in childhood medulloblastoma. Acta Neuropathol Commun 2:39. doi:10.1186/2051-5960-2-39
Kristoffersen K, Villingshøj M, Poulsen HS, Stockhausen M-T (2013) Level of Notch activation determines the effect on growth and stem cell-like features in glioblastoma multiforme neurosphere cultures. Cancer Biol Ther 14:625–637. doi:10.4161/cbt.24595
Taylor MD, Poppleton H, Fuller C et al (2005) Radial glia cells are candidate stem cells of ependymoma. Cancer Cell 8:323–335. doi:10.1016/j.ccr.2005.09.001
Jones DTW, Jäger N, Kool M et al (2012) Dissecting the genomic complexity underlying medulloblastoma. Nature 488:100–105. doi:10.1038/nature11284
Natarajan S, Li Y, Miller EE et al (2013) Notch1-induced brain tumor models the sonic hedgehog subgroup of human medulloblastoma. Cancer Res 73:5381–5390. doi:10.1158/0008-5472.CAN-13-0033
Ignatova TN, Kukekov VG, Laywell ED et al (2002) Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 39:193–206. doi:10.1002/glia.10094
Fan X, Khaki L, Zhu TS et al (2010) NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28:5–16. doi:10.1002/stem.254
LoRusso P, Demuth T (2009) E H Phase I study of the gamma secretase inhibitor MK-0752 in patients with metastatic breast and other advanced solid tumors. 100th Annual Meeting of the American Association for Cancer Research, Denver, CO, April 18–22 (abstr 3605)
Krop I, Demuth T, Guthrie T et al (2012) Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol 30:2307–2313. doi:10.1200/JCO.2011.39.1540
Schott AF, Landis MD, Dontu G et al (2013) Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res 19:1512–1524. doi:10.1158/1078-0432.CCR-11-3326
Fouladi M, Stewart CF, Olson J et al (2011) Phase I trial of MK-0752 in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J Clin Oncol 29:3529–3534. doi:10.1200/JCO.2011.35.7806
Skolnik JM, Barrett JS, Jayaraman B et al (2008) Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol 26:190–195. doi:10.1200/JCO.2007.12.7712
Bai F, Tagen M, Colotta C et al (2010) Determination of the gamma-secretase inhibitor MK-0752 in human plasma by online extraction and electrospray tandem mass spectrometry (HTLC-ESI-MS/MS). J Chromatogr B Analyt Technol Biomed Life Sci 878:2348–2352. doi:10.1016/j.jchromb.2010.07.019
Real PJ, Tosello V, Palomero T et al (2009) Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med 15:50–58. doi:10.1038/nm.1900
Pugh TJ, Weeraratne SD, Archer TC et al (2012) Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature 488:106–110. doi:10.1038/nature11329
Blackman SC, Podtelezhnikov A, Railkar RA Notch pathway inhibition with MK-0752 leads to dose- and time-dependent transcriptional alterations in proliferation, PI3K, and Wnt pathway genes in plucked human hair follicles. Proc Am Assoc for Cancer Res 51:(abst 26)
Balint K, Xiao M, Pinnix CC et al (2005) Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest 115:3166–3176. doi:10.1172/JCI25001
Wang T, Holt CM, Xu C et al (2007) Notch3 activation modulates cell growth behaviour and cross-talk to Wnt/TCF signalling pathway. Cell Signal 19:2458–2467. doi:10.1016/j.cellsig.2007.07.019
Brechbiel J, Miller-Moslin K, Adjei AA (2014) Crosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancer. Cancer Treat Rev 40:750–759. doi:10.1016/j.ctrv.2014.02.003
Tremblay I, Paré E, Arsenault D et al (2013) The MEK/ERK pathway promotes NOTCH signalling in pancreatic cancer cells. PLoS One 8:e85502. doi:10.1371/journal.pone.0085502
Hales EC, Taub JW, Matherly LH (2014) New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia. Cell Signal 26:149–161. doi:10.1016/j.cellsig.2013.09.021
Zhao N, Guo Y, Zhang M et al (2010) Akt-mTOR signaling is involved in Notch-1-mediated glioma cell survival and proliferation. Oncol Rep 23:1443–1447
Jin R, Nakada M, Teng L et al (2013) Combination therapy using Notch and Akt inhibitors is effective for suppressing invasion but not proliferation in glioma cells. Neurosci Lett 534:316–321. doi:10.1016/j.neulet.2012.12.008
Fouladi M, Perentesis JP, Phillips CL et al (2014) A phase I trial of MK-2206 in children with refractory malignancies: a Children’s Oncology Group study. Pediatr Blood Cancer 61:1246–1251. doi:10.1002/pbc.25023
Fouladi M, Laningham F, Wu J et al (2007) Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol 25:4806–4812. doi:10.1200/JCO.2007.11.4017
Gu F, Ma Y, Zhang Z et al (2010) Expression of Stat3 and Notch1 is associated with cisplatin resistance in head and neck squamous cell carcinoma. Oncol Rep 23:671–676
McAuliffe SM, Morgan SL, Wyant GA et al (2012) Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci U S A 109:E2939–48. doi:10.1073/pnas.1206400109
Groeneweg JW, DiGloria CM, Yuan J et al (2014) Inhibition of notch signaling in combination with Paclitaxel reduces platinum-resistant ovarian tumor growth. Front Oncol 4:171. doi:10.3389/fonc.2014.00171
Hiddingh L, Tannous BA, Teng J et al (2014) EFEMP1 induces γ-secretase/Notch-mediated temozolomide resistance in glioblastoma. Oncotarget 5:363–374
Acknowledgments
This study was funded in part by the National Institutes of Health Grant No. U01 CA81457 for the Pediatric Brain Tumor Consortium and by the American Lebanese Syrian Associated Charities. Maryam Fouladi received research funding by Merck.
Compliance with ethical standards
ᅟ
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoffman, L.M., Fouladi, M., Olson, J. et al. Phase I trial of weekly MK-0752 in children with refractory central nervous system malignancies: a pediatric brain tumor consortium study. Childs Nerv Syst 31, 1283–1289 (2015). https://doi.org/10.1007/s00381-015-2725-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2725-3