Abstract
Background
Reactive gliosis has been implicated in the pathogenesis of communicating hydrocephalus. Because the activation of Wnt/β-catenin signaling pathway is considered as a significant factor to contribute to brain development, neurodegenerative process, and reactive gliosis, we target this pathway for intervention by using sFRP-l and investigated the expression of β-catenin, cyclin D-1, and glial fibrillary acidic protein (GFAP) in the brain of experimental hydrocephalic rats in terms of protein and gene expression.
Methods
Therefore, 30 adult SD rats were randomly divided into the normal group (n = 5), the sham operation group (n = 5), the hydrocephalus group (n = 10), and the sFRP-l group (n = 10). Hydrocephalic rat models were induced by intraventricular injections of 3 % kaolin while sFRP-l group was treated by sFRP-l with kaolin injections. The ventricular dilatation was examinated by MRI at 2-week post-operation. After that, β-catenin, cyclin D-1, and GFAP were qualified by Western blot and immunohistochemistry.
Results
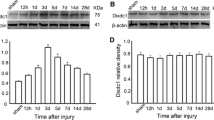
According to the result, the expression of β-catenin and cyclin D-1 increased (P < 0. 05) in the brain tissue of the hydrocephalus group compared with that of the sham group, while GFAP expression in the hydrocephalus group is more obvious (P < 0. 05). In the sFRP-l group, the expression of β-catenin and cyclin D-1 and GFAP expression is lower (P < 0. 05) compared with those of the hydrocephalus group. We demonstrated that the Wnt/β-catenin pathway is activated in the experimental hydrocephalic rat brain. sFRP-l inhibited the expression of β-catenin and cyclin D-1 and alleviated reactive gliosis in the hydrocephalic rat brain tissue, while the development of hydrocephalus was delayed.
Conclusion
These results suggest that regulating Wnt/β-catenin signaling pathway may provide new therapeutic methods for hydrocephalic patients.






Similar content being viewed by others
References
Aleksandra S, Rafal S, Lubomir B, Wojciech O, Marzena C, Wojciech K, Cezary S, Tadeusz P, Maciej W, Monika L (2011) Prognostic significance of Wnt-1, β-catenin and E-cadherin expression in advanced colorectal carcinoma. Pathol Oncol Res 17:955–963
Angadi PV, Krishnapillai R (2007) Cyclin D1 expression in oral squamous cell carcinoma and verrucous carcinoma: correlation with histological differentiation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:e30–5
Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10:468–477
Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA (1999) Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem 274:16180–16187
Barandon L, Casassus F, Leroux L, Moreau C, Allières C, Lamazière JM, Dufourcq P, Couffinhal T, Duplàa C (2011) Secreted frizzled-related protein-1 improves postinfarction scar formation through a modulation of inflammatory response. Arterioscler Thromb Vasc Biol 31(11):e80–7
Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, Sonnylal S, de Crombrugghe B, Taketo MM, Distler O, Schett G, Distler JH (2012) β-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis 71:761–7
Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J (2008) Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 121:737–46
Cherian S, Whitelaw A, Thoresen M, Love S (2004) The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol 14:305–11
Chien AJ, Conrad WH, Moon RT (2009) A Wnt survival guide: from flies to human disease. J Invest Dermatol 129:1614–1627
Clevers H, Nusse R (2012) Wnt/β-catenin signaling and disease. Cell 149:1192–205
Coyle-Rink J, Del Valle L, Sweet T, Khalili K, Amini S (2002) Developmental expression of Wnt signaling factors in mouse brain. Cancer Biol Ther 1:640–645
Deren KE, Packer M, Forsyth J, Milash B, Abdullah OM, Hsu EW, McAllister JP 2nd (2010) Reactive astrocytosis, microgliosis and inflammation in rats with neonatal hydrocephalus. Exp Neurol 226(1):110–9
Dufourcq P, Couffinhal T, Ezan J, Barandon L, Moreau C, Daret D, Duplaa C (2002) FrzA, a secreted frizzled related protein, induced angiogenic response. Circulation 106:3097–3103
Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamaziere JM, Couffinhal T, Duplaa C (2008) Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells 26:2991–3001
Glaw JT, Skalak TC, Peirce SM (2010) Inhibition of canonical Wnt signaling increases microvascular hemorrhaging and venular remodeling in adult rats. Microcirculation 17(5):348–57
Godin JD, Poizat G, Hickey MA, Maschat F, Humbert S (2010) Mutant huntingtin-impaired degradation of beta-catenin causes neurotoxicity in Huntington’s disease. EMBO J 29(14):2433–45
He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y (2009) Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20:765–776
He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF, Liu Y (2012) Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/beta-catenin activity in CKD. J Am Soc Nephrol 23:294–304
Huang H, He X (2008) Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol 20:119–125
Inestrosa NC, Varela-Nallar L, Grabowski CP, Colombres M (2007) Synaptotoxicity in Alzheimer’s disease: the Wnt signaling pathway as a molecular target. IUBMB Life 59(4–5):316–21
Kim TH, Kim SH, Seo JY, Chung H, Kwak HJ, Lee SK, Yoon HJ, Shin DH, Park SS, Sohn JW (2011) Blockade of the Wnt/β-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med 223:45–54
Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, Chapman HA (2009) Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 119:213–224
Li W, Zhu C, Chen X, Li Y, Gao R, Wu Q (2011) Pokeweed antiviral protein down-regulates Wnt/β-catenin signalling to attenuate liver fibrogenesis in vitro and in vivo. Dig Liver Dis 43:559–66
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagents. J Biol Chem 193:265–275
Macdonald BT, Semenov MV, He X (2007) SnapShot: Wnt/beta-catenin signaling. Cell 131:1204
McAllister JP 2nd, Miller JM (2010) Minocycline inhibits glial proliferation in the H-Tx rat model of congenital hydrocephalus. Cerebrospinal Fluid Res 27(7):7
Miller JM, Kumar R, McAllister JP 2nd, Krause GS (2006) Gene expression analysis of the development of congenital hydrocephalus in the H, Brain Res. 23;1075(1):36–47
Moon RT, Kohn AD, De Ferrari GV, Kaykas A (2004) WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 5(9):691–701
Nagra G, Li J, McAllister JP 2nd, Miller J, Wagshul M, Johnston M (2008) Impaired lymphatic cerebrospinal fluid absorption in a rat model of kaolin-induced communicating hydrocephalus. Am J Physiol Regul Integr Comp Physiol 294:R1752–1759
Nelson PJ, von Toerne C, Grane HJ (2011) Wnt-signaling pathways in progressive renal fibrosis. Expert Opin Ther Targets 15:1073–83
Panhuysen M, Vogt, Weisenhorn DM, Blanquet V, Brodski C, Heinzmann U, Beisker W, Wurst W (2004) Effects of Wnt1 signaling on proliferation in the developing mid-/hindbrain region. Mol Cell Neurosci 26:101–111
Pannitteri G, Marino B, Campa PP, Martucci R, Testa U, Peschle C (1997) Interleukins 6 and 8 as mediators of acute phase response in acute myocardial infarction. Am J Cardiol 80:622–625
Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G (2008) Wnt5a/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol 28:504–510
Pereira CP, Bachli EB, Schoedon G (2009) The Wnt pathway: a macrophage effector molecule that triggers inflammation. Curr Atheroscler Rep 11:236–242
Rekate HL (2011) A consensus on the classification of hydrocephalus: its utility in the assessment of abnormalities of cerebrospinal fluid dynamics. Childs Nerv Syst 27(10):1535–41
Shang YC, Wang SH, Xiong F, Zhao CP, Peng FN, Feng SW, Li MS, Li Y, Zhang C (2007) Wnt3a signaling promotes proliferation, myogenic differentiation, and migration of rat bone marrow mesenchymal stem cells. Acta Pharmacol Sin 28:1761–1774
Toledo EM, Colombres M, Inestrosa NC (2008) Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol 86(3):281–96
White BD, Nguyen NK, Moon RT (2007) Wnt signaling: it gets more humorous with age. Curr Biol 17:R923–R925
Xu H, Zhang SL, Tan GW, Zhu HW, Huang CQ, Zhang FF, Wang ZX (2012) Reactive gliosis and neuroinflammation in rats with communicating hydrocephalus. Neuroscience 218:317–325
Xu H, Wang Z, Zhang S, Tan G, Zhu H (2013) Procollagen type I C-terminal propeptide, procollagen type III N-terminal propeptide, hyaluronic acid, and laminin in the cerebrospinal fluid of rats with communicating hydrocephalus. J Neurosurg Pediatr 11(6):692–6
Yamamoto H, Awada C, Hanaki H, Sakane H, Tsujimoto I, Takahashi Y, Takao T, Kikuchi A (2013) Apicobasal secretion of Wnt11 and Wnt3a in polarized epithelial cells is regulated by distinct mechanisms. J Cell Sci 12:2013
Zhang L, Yang X, Yang S, Zhang J (2011) The Wnt/beta-catenin signaling pathway in the adult neurogenesis. Eur J Neurosci 33:1–8
Acknowledgments
This work was supported by the Natural Science Foundation of China 81271332.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hao Xu and Bin Xu equally contributed to this work.
Rights and permissions
About this article
Cite this article
Xu, H., Xu, B., Wang, Z. et al. Inhibition of Wnt/β-catenin signal is alleviated reactive gliosis in rats with hydrocephalus. Childs Nerv Syst 31, 227–234 (2015). https://doi.org/10.1007/s00381-014-2613-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-014-2613-2