Abstract
Purpose
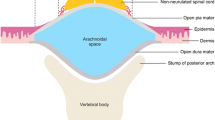
The prevention of Chiari type II malformation (CM) is commonly used as a primary outcome for the evaluation of techniques of fetal myelomeningocele (MMC) surgery in the fetal lamb. The aim of our study was to investigate the frequency of the associated CM in the MMC fetal sheep model and to investigate the contribution of prenatal ultrasound evaluation of CM at the time of prenatal repair.
Methods
A MMC-like lesion was surgically created at 75 days of gestation in 21 fetuses performing a L1–L5 laminectomy followed by an excision of the exposed dura and a midline myelotomy. At a 90-day gestation, among the 19 alived fetuses, a conventional repair of the MMC-like lesion was performed in seven, four of whom underwent cerebral ultrasound (US) examination before the repair. Twelve fetuses remained untreated (control group). All fetuses underwent post-mortem examination (PM) at 138 days.
Results
At a 90-day gestation, CM was demonstrated by US examination in all four evaluated fetuses. At birth, CM was found in 3/6 control whether CM was absent in all alived fetuses in the prenatal repair group (n = 4).
Conclusions
Creation of a MMC-like lesion with an additional myelotomy does not always lead to hindbrain herniation. Our study suggests that CM should be assessed by ultrasound examination at the time of the prenatal repair to demonstrate the effectiveness of new techniques for the prenatal repair of MMC.



Similar content being viewed by others
References
Heffez DS, Aryanpur J, Rotellini NA, Hutchins GM, Freeman JM (1993) Intrauterine repair of experimental surgically created dysraphism. Neurosurgery 32(6):1005–1010
Meuli M, Meuli-Simmen C, Yingling CD, Hutchins GM, Hoffman KM, Harrison MR, Adzick NS (1995) Creation of myelomeningocele in utero: a model of functional damage from spinal cord exposure in fetal sheep. J Pediatr Surg 30(7):1028–1032
Meuli M, Meuli-Simmen C, Yingling CD, Hutchins GM, Timmel GB, Harrison MR, Adzick NS (1996) In utero repair of experimental myelomeningocele spares neurologic function at birth. J Pediatr Surg 31(3):397–402
Paek BW, Farmer DL, Wilkinson CC, Albanese CT, Peacock W, Harrison MR, Jennings RW (2000) Hindbrain herniation develops in surgically created myelomeningocele but is absent after repair in fetal lambs. Am J Obstet Gynecol 183(5):1119–1123
Bouchard S, Davey MG, Rintoul NE, Walsh DS, Rorke LB, Adzick NS (2003) Correction of hindbrain herniation and anatomy of the vermis after in utero repair of myelomeningocele in sheep. J Pediatr Surg 38(3):451–458
Adzick NS, Thom EA, Spong CY, Brock JW 3rd, Burrows PK, Johnson MP et al (2011) A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364(11):993–1004. doi:10.1056/NEJMoa1014379
Fontecha CG, Peiro JL, Aguirre M, Soldado F, Añor S, Fresno L, Martinez-Ibañez V (2009) Inert patch with bioadhesive for gentle foetal surgery of myelomeningocele in a sheep model. Eur J Obstet Gynecol Reprod Biol 146(2):174–179. doi:10.1016/j.ejogrb.2009.06.022
Michejda M (1984) Intrauterine treatment of spina bifida : primate model. Z Kinderchir 39(4):259–261
Heffez DS, Aryanpur J, Hutchins GM, Freeman JM (1990) The paralysis associated with myelomeningocele : clinical and experimental data implicating a preventable spinal cord injury. Neurosurgery 26(6):987–992
Von Koch CS, Compagnone N, Hirose S, Yoder S, Harrison MR, Farmer DL (2005) Myelomeningocele: characterization of a surgically induced sheep model and its central nervous system similarities and differences to the human disease. Am J Obstet Gynecol 193(4):1456–1462
Encinas Hernández JL, Soto C, García-Cabezas MA, Pederiva F, Garriboli M, Rodríguez R, Peiró JL, Carceller F, López-Santamaría M, Tovar JA (2008) Brain malformations in the sheep model of myelomeningocele are similar to those found in human disease: preliminary report. Pediatr Surg Int 24(12):1335–1340. doi:10.1007/s00383-008-2276-8
Peiro JL, Fontecha CG, Ruano R, Esteves M, Fonseca C, Marotta M, Haeri S, Belfort MA (2013) Single-Access Fetal Endoscopy (SAFE) for myelomeningocele in sheep model I: amniotic carbon dioxide gas approach. Surg Endosc 27(10):3835–3840. doi:10.1007/s00464-013-2984-6
Acknowledgements
This study was supported by a grant from Fondation de l’Avenir pour la Recherche Médicale Appliquée (ETO-586).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guilbaud, L., Garabedian, C., Di Rocco, F. et al. Limits of the surgically induced model of myelomeningocele in the fetal sheep. Childs Nerv Syst 30, 1425–1429 (2014). https://doi.org/10.1007/s00381-014-2426-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-014-2426-3