Abstract
Objective
Our objective was to investigate the relation between the embryological development and neural tissue maturation at the site where the neural plate failed to form a neural tube.
Material and methods
Samples from 15 aborted human fetuses with neural tube defects (NTD). All of the fetuses were between 20 and 25 gestational weeks old. Indicators of neural tissue maturation, formation of basal lamina, expression of integrins and neuron specific class III beta tubulin (tuj1) were investigated. To detect the adverse effects of the environment, if any, p53 and bcl-2 activity at both sites of the open and closed neural plate were investigated as well.
Results
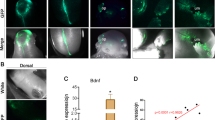
No difference was found in the expression of maturation-related molecules at the site of the neural plate that remained open compared with the site where the neural tube is normally formed. While high p53 activity was noted in neural tissue at the site of the neural tube defect, no such activity was detected in the neural tissue where the neural tube is normally formed.
Conclusion
Our results suggested that maturation and differentiation of neural tissue continued regardless of the failure of neural tube closure. Therefore, the neurological deficits that are encountered in NTD patients should be related to secondary damage such as amnion fluid toxicity, uterus contractions, labor, etc. It seems valuable to save the neural plate before the negative effects of the environment renders the neural tissue functionless.








Similar content being viewed by others
References
Dalton SL, Scharf E, Briesewitz R, Marcatonio EE, Assoian RK (1995) Cell adhesion to extracellular matrix regulates the life cycle of integrins. Development 6:1781–1791
Edgar D, Timpl R, Thoenen H (1984) Structural requirements for the stimulation of neurite outgrowth by two variants of laminin and their inhibition by antibodies. J Cell Biol 106:1299–1306
Giancotti FG, Ruoslahti E (1999) Integrin signalling. Science 285:1028–1032
Grossmann J (2002) Molecular mechanisms of “detachment-induced apoptosis-Anoikis”. Apoptosis 7:247–260
Klein G, Langegger M, Timpl R, Ekblom P (1998) Role of the laminin a chain in the development of epithelial cell polarity. Cell 55:331–341
Martin GR, Timpl R (1987) Laminin and other basement membrane components. Annu Rev Cell Biol 3:57–85
McLone DG, Dias MS, Goossens W, Knepper PA (1997) Pathological changes in exposed neural tissue of fetal delayed splotch (Spd) mice. Childs Nerv Syst 13:1–7
Meredith JE Jr, Winitz S, Lewis JM et al (1996) The regulation of growth and intracellular signalling by integrins. Endocr Rev 17:207–220
Meuli M, Meuli-Simmen C, Hutchins GM, Yingling CD, Hofmann KM, Harrison MR, Adzick NS (1995) In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat Med 1:342–347
Meuli M, Meuli-Simmen C, Yingling CD, Hutchins GM, Timmel GB, Harrison MR, Adzick NS (1996) In utero repair of experimental myelomeningocele saves neurological function at birth. J Pediatr Surg 31:397–402
Selçuki M, Manning S, Bernfield B (2001) Curly tail mouse model of human neural tube defects demonstrates normal spinal cord differentiation at the level of meningomyelocele: implications for fetal surgery. Childs Nerv Syst 17:19–23
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selçuki, M., Vatansever, S., Inan, S. et al. Neural tissue continues its maturation at the site of neural tube closure defects: implications for prenatal intervention in human samples. Childs Nerv Syst 20, 313–320 (2004). https://doi.org/10.1007/s00381-004-0950-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-004-0950-2