Abstract
Introduction
Subdural hygroma is a known complication of Sylvian fissure arachnoid cysts (SACs). However, most of the data in the literature refer to spontaneous or posttraumatic occurrence of subdural hygromas, regarded as either a favorable or an unfavorable event. Little is known about this phenomenon as a consequence of the surgical management of SACs. The present study was carried out to evaluate the significance of postoperative subdural hygromas in children with temporal arachnoid cysts, who have been treated with craniotomy and wide marsupialization of the cystic membrane.
Clinical material and results
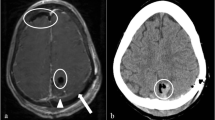
Between 1980 and March 2002, 104 children were operated on for a SAC at the Pediatric Neurosurgical Unit of the Catholic University Medical School in Rome. Six patients (5.8%; boys/girls=4/2; mean age 3.28 years) developed a subdural hygroma postoperatively, which required further treatment. According to Galassi's classification 4 children had a Type III cyst and 2 children a Type II cyst. All the patients had previously been submitted to open marsupialization of their arachnoid cyst and extensive removal of the cyst wall. In 5 patients (Type III cyst: 4 patients; Type II cyst: 1 patient), acute or subacute symptoms and signs of increased intracranial pressure (ICP) developed at a temporal distance, varying from 5 days to 1 month (mean interval: 14.3 days). All these children needed surgical treatment for the hygroma. A subduro-peritoneal (SDP) shunt was implanted as the first step in 3 patients (Type III cyst: 2 patients; Type II cyst: 1 patient). In the remaining 2 patients of this group (Type III cysts) medical therapy was initially attempted (acetazolamide: 24 mg/kg) without any improvement in clinical manifestations; on these grounds an external spinal subarachnoid drainage was implanted, but led only to the transient clearance of symptoms. The direct drainage of the subdural collection (SDP shunt in 1 patient and temporary external subdural drainage in the other), was followed by complete clinical recovery in both cases. The last patient in this series showed a pathologic increase in head circumference during the 1st month after surgery for his arachnoid cyst, followed by a subsequent stabilization. A CT scan documented a subdural hygroma, which first of all increased in size, but stabilized 2 months after surgery. No surgical treatment was performed in this case. At a mean follow-up of 2.38 years (minimum: 1 month; maximum: 5 years) all the patients are in excellent clinical conditions. Complete neuroradiological resolution of the hygroma was observed in 2 of the 4 shunted patients, who have both been submitted to shunt removal (2 and 3 years after the implant respectively).
Conclusions
It is our opinion that a wide opening of the external membrane of SACs may predispose the CSF fluid to accumulate within the subdural space, where its absorption is insufficient. A ball mechanism created by CSF pulsation may further increase the subdural fluid accumulation. Secondary distortion and occlusion at the level of the basal cisterns may also contribute to the persistence of the subdural fluid collection. Clinical manifestations may be transient, but frequently have a progressive course and the drainage of the subdural collection is, though transient, required in most cases. On these grounds we suggest the selective opening of the basal cisterns associated with a limited resection of the outer cyst walls in order to limit CSF access to the subdural space.


Similar content being viewed by others
References
Albuquerque FC, Giannotta SL (1997) Arachnoid cyst rupture producing subdural hygroma and intracranial hypertension: case reports. Neurosurgery 41:951–956
Arai H, Sato K, Wachi A, Okuda O, Takeda N (1996) Arachnoid cysts of the middle cranial fossa: experience with 77 patients who were treated with cystoperitoneal shunting. Neurosurgery 39:1108–1113
Beltramello A, Mazza C (1985) Spontaneous disappearance of a large middle fossa arachnoid cyst. Surg Neurol 24:181–183
Caldarelli M, Di Rocco C, Romani R (2002) Surgical treatment of chronic subdural hygromas in infants and children. Acta Neurochir 144:581–588
Cayli SR (2000) Arachnoid cyst with spontaneous rupture into the subdural space. Br J Neurosurg 14:568–570
Choong CT, Lee SH (1998) Subdural hygroma in association with middle fossa arachnoid cyst: acetazolamide therapy. Brain Dev 20:319–322
Cullis PA, Gilroy J (1983) Arachnoid cyst with rupture into the subdural space. J Neurol Neurosurg Psychiatry 46:454–456
Di Rocco C (1996) Arachnoid cysts. In: Youmans JR (ed) Neurological surgery, vol 2. Saunders, pp 967–994
Donaldson JW, Edwards-Brown M, Luerssen TG (2000) Arachnoid cyst rupture with concurrent subdural hygroma. Pediatr Neurosurg 32:137–139
Fewel ME, Levy ML, McComb JG (1996) Surgical treatment of 95 children with 102 intracranial arachnoid cysts. Pediatr Neurosurg 25:165–173
Galassi E, Tognetti F, Gaist G, Fagioli L, Frank F, Frank G (1982) CT and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: classification and pathophysiological aspects. Surg Neurol 17:362–369
Gelabert-Gonzàlez M, Fernandez-Villa J, Cutrin Prieto J, Garcia Allut A, Martinez-Rumbo R (2002) Arachnoid cyst rupture with subdural hygroma. Report of three cases and literature review. Childs Nerv Syst 18:609–613
Inoue T, Matsushima T, Tashima S, Fukui M, Hasuo K (1987) Spontaneous disappearance of a middle fossa arachnoid cyst associated with subdural hematoma. Surg Neurol 28:447–450
Kang JK, Lee KS, Lee IW, Jeun SS, Son BC, Yung CK, Park YS, Lee SW (2000) Shunt-independent surgical treatment of middle cranial fossa arachnoid cysts in children. Childs Nerv Syst 16:111–116
Mori T, Fujimoto M, Sakae K, Takehiko S, Shin H, Yamaki T, Ueda S (1995) Disappearance of arachnoid cysts after head injury. Neurosurgery 36:938–942
Motoyama Y, Nabeshima S, Yamazoe N, Isaka F, Higuchi K, Satow T (2001) Surgical treatment for symptomatic arachnoid cysts. No Shinkei Geka 29:217–226
Oberbauer RW, Haase J, Pucher R (1992) Arachnoid cysts in children: a European co-operative study. Childs Nerv Syst 8:281–286
Olsen NK, Madsen HHT (1990) Arachnoid cyst with complicating intracystic and subdural hemorrhage. Rontgenblatter 43:166–168
Parsch CS, Kraub J, Hofmann E, Meixensberger J, Roosen K (1997) Arachnoid cysts associated with subdural hematomas and hygromas: analysis of 16 cases, long-term follow-up, and review of the literature. Neurosurgery 40:483–490
Rakier A, Feinsod M (1995) Gradual resolution of an arachnoid cyst after spontaneous rupture into the subdural space. J Neurosurg 83:1085–1086
Sener RN (1997) Arachnoid cysts associated with post-traumatic and spontaneous rupture into the subdural space. Comput Med Imaging Graph 21:341–344
Stein SC (1981) Intracranial developmental cysts in children: treatment by cystoperitoneal shunting. Neurosurgery 8:647–650
Verna TRK, Sulzimir CB, Miles JP (1981) Posttraumatic complication of arachnoid cysts and temporal lobe agenesis. J Neurol Neurosurg Psychiatry 44:29–34
Yamanouchi Y, Someda K, Oka N (1986) Spontaneous disappearance of middle fossa arachnoid cyst after head injury. Childs Nerv Syst 2:40–43
Yokoyama K, Tonami N, Kimurra M, Kiroshita A, Aburrano T, Hiseda K (1989) Scintigraphic demonstration of intracranial communication between arachnoid cyst and associated hematoma. Clin Neurol Med 14:350–353
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamburrini, G., Caldarelli, M., Massimi, L. et al. Subdural hygroma: an unwanted result of Sylvian arachnoid cyst marsupialization. Childs Nerv Syst 19, 159–165 (2003). https://doi.org/10.1007/s00381-003-0724-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-003-0724-2