Abstract
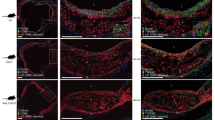
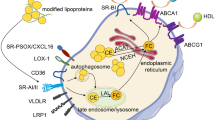
Genetic lineage tracing studies have shown that phenotypic switching of vascular smooth muscle cells (VSMCs) results in less-differentiated cells, including macrophage-like cells that lack traditional VSMC markers. This switching contributes to the formation of necrotic core in plaques and promotes atherosclerosis, which is important for plaque stability. Niclosamide, a commonly used anti-helminthic drug, has recently attracted attention as an anti-cancer drug that inhibits multiple signaling pathways. The expression of the S100A4 protein is upregulated in synthetic VSMCs and inhibited by niclosamide on metastatic progression in colon cancer. We aimed to test the effect of niclosamide on VSMC phenotype switching and plaque stability. To examine murine atherosclerosis, we induced experimental lesions by blood flow cessation in apolipoprotein E knockout mice fed a high-fat diet. Oral administration of niclosamide changed 4-week-old plaques to collagen-rich and less-necrotic core phenotypes and downregulated the expression of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in vivo. In vitro analysis indicated that niclosamide reduced LOX-1 expression in VSMCs in a concentration-dependent and S100A4-independent manner. The inhibitory effect of niclosamide on LOX-1 and collagen type I was associated with the inactivation of the nuclear factor-κB signaling pathway. We demonstrated that the administration of niclosamide reduced LOX-1 expression and altered the composition of murine carotid plaques. Our results highlight the potential of niclosamide as an atheroprotective agent that enhances atherosclerotic plaque stability.






Similar content being viewed by others
Abbreviations
- VSMCs:
-
Vascular smooth muscle cells
- LOX-1:
-
Lectin-like oxidized low-density lipoprotein receptor-1
- NF-κB:
-
Nuclear factor-κB
- SMA:
-
Smooth muscle actin
- PL:
-
Partial ligation
- CA:
-
Carotid artery
- ApoE:
-
Apolipoprotein E
- IEL:
-
Internal elastic lamina
- MβCD:
-
Methyl-β-cyclodextrin
- LPS:
-
Lipopolysaccharide
- ox-LDL:
-
Oxidized low-density lipoprotein
- ROS:
-
Reactive oxygen species
- AMPK:
-
AMP-activated protein kinase
References
Hansson GK, Libby P, Tabas I (2015) Inflammation and plaque vulnerability. J Intern Med 278(5):483–493
Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84(3):767–801
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362(6423):801–809
Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R (2014) Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res 115(7):662–667
Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21(6):628–637
Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA (2014) Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129(15):1551–1559
Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA (2015) Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol 35(3):535–546
Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W (2005) Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol 25(6):1256–1261
Moore KJ, Freeman MW (2006) Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol 26(8):1702–1711
Naito M, Suzuki H, Mori T, Matsumoto A, Kodama T, Takahashi K (1992) Coexpression of type I and type II human macrophage scavenger receptors in macrophages of various organs and foam cells in atherosclerotic lesions. Am J Pathol 141(3):591–599
Li W, Febbraio M, Reddy SP, Yu DY, Yamamoto M, Silverstein RL (2010) CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J Clin Invest 120(11):3996–4006
Aoyama T, Chen M, Fujiwara H, Masaki T, Sawamura T (2000) LOX-1 mediates lysophosphatidylcholine-induced oxidized LDL uptake in smooth muscle cells. FEBS Lett 467(2–3):217–220
Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T (1997) An endothelial receptor for oxidized low-density lipoprotein. Nature 386(6620):73–77
Draude G, Hrboticky N, Lorenz RL (1999) The expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) on human vascular smooth muscle cells and monocytes and its down-regulation by lovastatin. Biochem Pharmacol 57(4):383–386
Li D, Mehta JL (2009) Intracellular signaling of LOX-1 in endothelial cell apoptosis. Circ Res 104(5):566–568
Tarabykina S, Griffiths TR, Tulchinsky E, Mellon JK, Bronstein IB, Kriajevska M (2007) Metastasis-associated protein S100A4: spotlight on its role in cell migration. Curr Cancer Drug Targets 7(3):217–228
Chaabane C, Coen M, Bochaton-Piallat ML (2014) Smooth muscle cell phenotypic switch: implications for foam cell formation. Curr Opin Lipidol 25(5):374–379
Nagata M, Minami M, Yoshida K, Yang T, Yamamoto Y, Takayama N, Ikedo T, Hayashi K, Miyata T, Yokode M, Miyamoto S (2020) Calcium-binding protein S100A4 is upregulated in carotid atherosclerotic plaques and contributes to expansive remodeling. J Am Heart Assoc. https://doi.org/10.1161/JAHA.120.016128
Sack U, Walther W, Scudiero D, Selby M, Kobelt D, Lemm M, Fichtner I, Schlag PM, Shoemaker RH, Stein U (2011) Novel effect of antihelminthic Niclosamide on S100A4-mediated metastatic progression in colon cancer. J Natl Cancer Inst 103(13):1018–1036
Li Y, Li PK, Roberts MJ, Arend RC, Samant RS, Buchsbaum DJ (2014) Multi-targeted therapy of cancer by niclosamide: a new application for an old drug. Cancer Lett 349(1):8–14
Ye T, Xiong Y, Yan Y, Xia Y, Song X, Liu L, Li D, Wang N, Zhang L, Zhu Y, Zeng J, Wei Y, Yu L (2014) The anthelmintic drug niclosamide induces apoptosis, impairs metastasis and reduces immunosuppressive cells in breast cancer model. PLoS One. https://doi.org/10.1371/journal.pone.0085887
Jin Y, Lu Z, Ding K, Li J, Du X, Chen C, Sun X, Wu Y, Zhou J, Pan J (2010) Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res 70(6):2516–2527
Tao H, Zhang Y, Zeng X, Shulman GI, Jin S (2014) Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat Med 20(11):1263–1269
Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H (2009) Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol 297(4):H1535-1543
Ohayon J, Finet G, Gharib AM, Herzka DA, Tracqui P, Heroux J, Rioufol G, Kotys MS, Elagha A, Pettigrew RI (2008) Necrotic core thickness and positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol 295(2):H717-727
Andres-Manzano MJ, Andres V, Dorado B (2015) Oil red O and hematoxylin and eosin staining for quantification of atherosclerosis burden in mouse aorta and aortic root. Methods Mol Biol 1339:85–99
Whittaker P, Kloner RA, Boughner DR, Pickering JG (1994) Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol 89(5):397–410
Ivan E, Khatri JJ, Johnson C, Magid R, Godin D, Nandi S, Lessner S, Galis ZS (2002) Expansive arterial remodeling is associated with increased neointimal macrophage foam cell content: the murine model of macrophage-rich carotid artery lesions. Circulation 105(22):2686–2691
Varghese F, Bukhari AB, Malhotra R, De A (2014) IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. https://doi.org/10.1371/journal.pone.0096801
Stoll LL, Denning GM, Weintraub NL (2004) Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol 24(12):2227–2236
Nagase M, Abe J, Takahashi K, Ando J, Hirose S, Fujita T (1998) Genomic organization and regulation of expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) gene. J Biol Chem 273(50):33702–33707
Shibata Y, Kume N, Arai H, Hayashida K, Inui-Hayashida A, Minami M, Mukai E, Toyohara M, Harauma A, Murayama T, Kita T, Hara S, Kamei K, Yokode M (2007) Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis 193(1):20–27
Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T (2000) Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem 275(17):12633–12638
Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P (2013) LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell Mol Life Sci 70(16):2859–2872
Chen W, Mook RA Jr, Premont RT, Wang J (2018) Niclosamide: beyond an antihelminthic drug. Cell Signal 41:89–96
Wieland A, Trageser D, Gogolok S, Reinartz R, Hofer H, Keller M, Leinhaas A, Schelle R, Normann S, Klaas L, Waha A, Koch P, Fimmers R, Pietsch T, Yachnis AT, Pincus DW, Steindler DA, Brustle O, Simon M, Glas M, Scheffler B (2013) Anticancer effects of niclosamide in human glioblastoma. Clin Cancer Res 19(15):4124–4136
Xiao XL, Hu N, Zhang XZ, Jiang M, Chen C, Ma R, Ma ZG, Gao JL, Xuan XC, Sun ZJ, Dong DL (2018) Niclosamide inhibits vascular smooth muscle cell proliferation and migration and attenuates neointimal hyperplasia in injured rat carotid arteries. Br J Pharmacol 175(10):1707–1718
Aoki C, Hattori Y, Tomizawa A, Jojima T, Kasai K (2010) Anti-inflammatory role of cilostazol in vascular smooth muscle cells in vitro and in vivo. J Atheroscler Thromb 17(5):503–509
Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat PL, Thomas M, Sawamura T (2007) Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res 100(11):1634–1642
Stein S, Lohmann C, Schafer N, Hofmann J, Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Boren J, McBurney MW, Landmesser U, Luscher TF, Matter CM (2010) SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur Heart J 31(18):2301–2309
Xia X, Li Y, Su Q, Huang Z, Shen Y, Li W, Yu C (2015) Inhibitory effects of Mycoepoxydiene on macrophage foam cell formation and atherosclerosis in ApoE-deficient mice. Cell Biosci 5(1):23
Rayner KJ (2017) Cell death in the vessel wall: the good, the bad, the ugly. Arterioscler Thromb Vasc Biol 37(7):e75–e81
Chang X, Zhen X, Liu J, Ren X, Hu Z, Zhou Z, Zhu F, Ding K, Nie J (2017) The antihelmenthic phosphate niclosamide impedes renal fibrosis by inhibiting homeodomain-interacting protein kinase 2 expression. Kidney Int 92(3):612–624
Ghebes CA, van Lente J, Post JN, Saris DB, Fernandes H (2017) High-throughput screening assay identifies small molecules capable of modulating the BMP-2 and TGF-beta1 signaling pathway. SLAS Discov 22(1):40–50
Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J (2001) Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 103(7):926–933
Rippe RA, Schrum LW, Stefanovic B, Solis-Herruzo JA, Brenner DA (1999) NF-kappaB inhibits expression of the alpha1(I) collagen gene. DNA Cell Biol 18(10):751–761
Roebuck KA, Vermes C, Carpenter LR, Fritz EA, Narayanan R, Glant TT (2001) Down-regulation of procollagen alpha1[I]] messenger RNA by titanium particles correlates with nuclear factor kappaB (NF-kappaB) activation and increased rel A and NF-kappaB1 binding to the collagen promoter. J Bone Miner Res 16(3):501–510
Acknowledgements
None.
Funding
This work was supported in part by grants from the Japan Society for the Promotion of Science JSPS KAKENHI: Grants No. 26460338 and 17K08592 (to MM) and 15K10299 and 20K08446 (to KY).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, T., Minami, M., Yoshida, K. et al. Niclosamide downregulates LOX-1 expression in mouse vascular smooth muscle cells and changes the composition of atherosclerotic plaques in ApoE−/− mice. Heart Vessels 37, 517–527 (2022). https://doi.org/10.1007/s00380-021-01983-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01983-z