Abstract
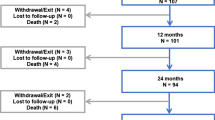
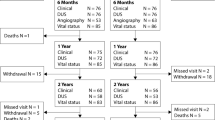
The RANGER II SFA objective was to evaluate the safety and effectiveness of the Ranger Drug-Coated Balloon (DCB) for treating superficial femoral artery and/or proximal popliteal artery lesions; the purpose of this cohort analysis is to assess the results among Japanese study participants. Patients eligible for RANGER II SFA had symptomatic lower limb ischemia (Rutherford classification 2–4) and were randomly assigned (3:1) to treatment with the Ranger DCB or standard percutaneous transluminal angioplasty (PTA). At 12 months, assessments included freedom from major adverse events (i.e., target lesion revascularization, major amputations, or death within 1 month of the index procedure) and core laboratory-assessed primary patency. Japanese patients (n = 102) comprised 27.1% of the overall study sample. Mean lesion lengths were 79.5 ± 44.0 mm and 84.0 ± 56.8 mm among Japanese patients treated with Ranger DCB (n = 77) or PTA (n = 25), respectively. All major adverse events were clinically driven TLRs (6.6% [5/76] for Ranger DCB and 16.0% [4/25] for PTA; p = 0.2194). Kaplan–Meier estimates of primary patency were 89.3% and 72.0%, respectively, at 12 months (log-rank p = 0.2134). Japanese patients treated with Ranger DCB maintained a high patency rate through 12 months and a low re-intervention rate.
Trial registration clinicaltrials.gov identifier: NCT03064126.



Similar content being viewed by others
References
Steiner S, Willfort-Ehringer A, Sievert H, Geist V, Lichtenberg M, Del Giudice C, Sauguet A, Diaz-Cartelle J, Marx C, Strobel A, Schult I, Scheinert D (2018) 12-Month results from the first-in-human randomized study of the ranger paclitaxel-coated balloon for femoropopliteal treatment. JACC Cardiovasc Interv 11:934–941
Steiner S, Schmidt A, Zeller T, Tepe G, Thieme M, Maiwald L, Schroder H, Euringer W, Ulrich M, Brechtel K, Brucks S, Blessing E, Schuster J, Langhoff R, Schellong S, Weiss N, Scheinert D (2020) COMPARE: prospective, randomized, non-inferiority trial of high- vs. low-dose paclitaxel drug-coated balloons for femoropopliteal interventions. Eur Heart J 41:2541–2552
Krishnan P, Faries P, Niazi K, Jain A, Sachar R, Bachinsky WB, Cardenas JA, Werner M, Brodmann M, Mustapha JA, Mena-Hurtado CI, Jaff MR, Holden AH, Lyden SP (2017) Stellarex drug-coated balloon for treatment of femoropopliteal disease: 12-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation 136:1102–1113
Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R, Muller-Hulsbeck S, Nehler MR, Benenati JF, Scheinert D (2015) Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med 373:145–153
Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR (2015) Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and/or popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation 131:495–502
Bausback Y, Willfort-Ehringer A, Sievert H, Geist V, Lichtenberg M, Del Giudice C, Sauguet A, Diaz-Cartelle J, Marx C, Ströbel A, Schult I, Scheinert D (2017) Six-month results from the initial randomized study of the ranger paclitaxel-coated balloon. J Endovasc Ther 24:459–467
Lichtenberg M, von Bilderling P, Ranft J, Niemoller K, Grell H, Briner L, Saucy F, Rassaf T, Breuckmann F (2018) Treatment of femoropopliteal atherosclerotic lesions using the ranger paclitaxel-coated balloon catheter: 12-month results from an all-comers registry. J Cardiovasc Surg (Torino) 59:45–50
Sachar R, Soga Y, Ansari MM, Lopez L, Brodmann M, Schroe H, Ramanath V, Diaz-Cartelle J, Zeller T (2021) 1-Year results from the RANGER II SFA randomized trial of the ranger drug-coated balloon. JACC Cardiovasc Interv 14:1123–1133
Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN (1997) Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 26:517–538
Iida O, Soga Y, Urasawa K, Saito S, Jaff MR, Wang H, Ookubo H, Yokoi H, Investigators M-SJ (2018) Drug-coated balloon vs standard percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal arteries: one-year results of the MDT-2113 SFA Japan Randomized Trial. J Endovasc Ther 25:109–117
Pharmaceuticals and Medical Devices Agency (PMDA) Review Summary Report—Lutonix (2017). https://www.pmda.go.jp/medical_devices/2017/M20170830001/780045000_22900BZX00252000_A100_1.pdf. Accessed March 2021
Acknowledgements
Analyses of the Japan cohort of the RANGER II SFA study were presented at the Annual Meeting of the Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT 2020, online). The authors thank the following Boston Scientific employees for their assistance: Hiroshi Sakamoto, Urara Yoshida, and Naoko Takahashi provided clinical trial management, and Elizabeth J. Davis, PhD provided medical writing assistance.
Funding
This study was funded by Boston Scientific Corporation, Marlborough, MA, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yoshimitsu Soga is an advisor to Boston Scientific. Masahiko Fujihara reports consulting for Boston Scientific. Yoshito Yamamoto reports no conflicts of interest. Shigeru Nakamura reports consulting for Asahi Intecc, OrbusNeich, Nipro, and Boston Scientific and receiving honoraria from Terumo, Medtronic, Medikit, Abbott. Osamu Iida reports consulting for Boston Scientific. Daizo Kawasaki reports consulting for Boston Scientific. Kazushi Urasawa reports consulting for Boston Scientific. Hiroshi Ando reports no conflicts of interest. Shinsuke Mori reports no conflicts of interest. Kenji Suzuki reports no conflicts of interest. Kazunori Horie reports no conflicts of interest. Juan Diaz-Cartelle is an employee of and owns stock in Boston Scientific. Amane Kozuki reports no relationships to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soga, Y., Fujihara, M., Yamamoto, Y. et al. One-year results for Japanese patients in RANGER II SFA. Heart Vessels 37, 568–573 (2022). https://doi.org/10.1007/s00380-021-01947-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01947-3