Abstract
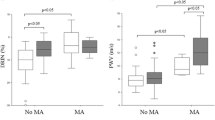
The renal arterial resistance index (RI) and the brachial–ankle pulse wave velocity (baPWV) are known as indicators of renal vascular resistance/systemic vascular damage and systemic arterial stiffness. The clinical significance of those parameters on clinical outcomes is poorly known in patients with and without heart failure with preserved ejection fraction (HFpEF). Baseline clinical data and the RI assessed by renal Doppler data, baPWV were obtained in patients with (HFpEF group, n = 60) and without HFpEF (non-HFpEF group, n = 51) who had a reduced estimated glomerular filtration rate (eGFR) of > 30 and < 60 mL/min/1.73 m2). We investigated the association between the RI and baPWV and major clinical outcomes including hospitalization for heart failure, cardiovascular death, myocardial infarction or unstable angina or other cardiovascular events and death from another cause. The RI and baPWV were greater in the HFpEF group than in the non-HF group (0.75 ± 0.07 vs. 0.69 ± 0.08, p < 0.001; 2002 ± 430 vs. 1762 ± 300 cm/s, p = 0.001). The RI correlated significantly with baPWV in the HFpEF (r = 0.382, p = 0.003) and non-HFpEF groups (r = 0.414, p = 0.002). During the median follow-up period of 54 months, major clinical outcomes occurred in 41 (36.9%) patients. The RI value, statin use and the presence of HFpEF were major factors for predicting clinical outcomes by multivariate analysis. Among the patients who had mild-to-moderate renal dysfunction, an increased RI and baPWV were observed in HFpEF patients as compared to non-HFpEF patients, but the baPWV similarly correlated with the RI value regardless of HFpFE patients or not. The strong association between the high RI value and presence of HFpEF and major clinical outcomes, suggests that not only the presence of HFpEF but also the high RI value may help to identify the high-risk patients leading to poor clinical outcomes.



Similar content being viewed by others
References
Redfield MM (2016) Heart failure with preserved ejection fraction. N Engl J Med 375(19):1868–1877
Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ (2016) Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 134(1):73–90
Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA (2007) Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 49(2):198–207
Kawaguchi M, Hay I, Fetics B, Kass DA (2003) Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 107(5):714–720
Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM (2009) Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 53(13):1119–1126
Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW (2013) Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci 68(8):968–975
Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CS, Navis G, Voors AA (2016) Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail 18(6):588–598
Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S (2003) Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—a survey of 12517 subjects. Atherosclerosis 166(2):303–309
Norris CS, Barnes RW (1984) Renal artery flow velocity analysis: a sensitive measure of experimental and clinical renovascular resistance. J Surg Res 36(3):230–236
Tublin ME, Tessler FN, Murphy ME (1999) Correlation between renal vascular resistance, pulse pressure, and the resistive index in isolated perfused rabbit kidneys. Radiology 213(1):258–264
Geraci G, Mule G, Geraci C, Mogavero M, D’Ignoto F, Morreale M, Foraci AC, Cottone S (2015) Association of renal resistive index with aortic pulse wave velocity in hypertensive patients. Eur J Prev Cardiol 22(4):415–422
Geraci G, Mule G, Mogavero M, Geraci C, D’Ignoti D, Guglielmo C, Cottone S (2015) Renal haemodynamics and severity of carotid atherosclerosis in hypertensive patients with and without impaired renal function. Nutr Metab Cardiovasc Dis 25(2):160–166
Lee WH, Hsu PC, Chu CY, Chen SC, Lee HH, Chen YC, Lee MK, Lee CS, Yen HW, Lin TH, Voon WC, Lai WT, Sheu SH, Kuo PL, Su HM (2018) Association of renal systolic time intervals with brachial-ankle pulse wave velocity. Int J Med Sci 15(11):1235–1240
Calabia J, Torguet P, Garcia I, Martin N, Mate G, Marin A, Molina C, Valles M (2014) The relationship between renal resistive index, arterial stiffness, and atherosclerotic burden: the link between macrocirculation and microcirculation. J Clin Hypertens (Greenwich) 16(3):186–191
Geraci G, Mule G, Paladino G, Zammuto MM, Castiglia A, Scaduto E, Zotta F, Geraci C, Granata A, Mansueto P, Cottone S (2017) Relationship between kidney findings and systemic vascular damage in elderly hypertensive patients without overt cardiovascular disease. J Clin Hypertens (Greenwich) 19(12):1339–1347
Pfeffer MA, Shah AM, Borlaug BA (2019) Heart failure with preserved ejection fraction in perspective. Circ Res 124(11):1598–1617
Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, Rizkala A, Lukashevich I, O’Meara E, Ryan JJ, Shah SJ, Mullens W, Zile MR, Lam CSP, McMurray JJV, Solomon SD, Investigators P-H (2019) Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 74(23):2858–2873
McKee PA, Castelli WP, McNamara PM, Kannel WB (1971) The natural history of congestive heart failure: the Framingham study. N Engl J Med 285(26):1441–1446
Aizawa Y, Sakata Y, Mano T, Takeda Y, Ohtani T, Tamaki S, Omori Y, Tsukamoto Y, Hirayama A, Komuro I, Yamamoto K (2011) Transition from asymptomatic diastolic dysfunction to heart failure with preserved ejection fraction: roles of systolic function and ventricular distensibility. Circ J 75(3):596–602
Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Ide T, Takeshita A, Tsutsui H (2009) Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 73(8):1442–1447
Izumi M, Sugiura T, Nakamura H, Nagatoya K, Imai E, Hori M (2000) Differential diagnosis of prerenal azotemia from acute tubular necrosis and prediction of recovery by Doppler ultrasound. Am J Kidney Dis 35(4):713–719
Takeda Y, Sakata Y, Higashimori M, Mano T, Nishio M, Ohtani T, Hori M, Masuyama T, Kaneko M, Yamamoto K (2009) Noninvasive assessment of wall distensibility with the evaluation of diastolic epicardial movement. J Card Fail 15(1):68–77
Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL Jr, Ribeiro LG, Miller RR (1981) A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation 64(4):744–753
Vuille C, Wayman AE (1994) Left ventricle I: general considerations, assessment of chamber size and function. In: Weyman AE (ed) Principles and practice of echocardiograpy, 2nd edn. Lea & Febiger, Philadelphia, pp 575–624
Bude RO, Rubin JM (1999) Relationship between the resistive index and vascular compliance and resistance. Radiology 211(2):411–417
Platt JF (1992) Duplex Doppler evaluation of native kidney dysfunction: obstructive and nonobstructive disease. AJR Am J Roentgenol 158(5):1035–1042
Ennezat PV, Marechaux S, Six-Carpentier M, Pincon C, Sediri I, Delsart P, Gras M, Mounier-Vehier C, Gautier C, Montaigne D, Jude B, Asseman P, Le Jemtel TH (2011) Renal resistance index and its prognostic significance in patients with heart failure with preserved ejection fraction. Nephrol Dial Transplant 26(12):3908–3913
Galesic K, Brkljacic B, Sabljar-Matovinovic M, Morovic-Vergles J, Cvitkovic-Kuzmic A, Bozikov V (2000) Renal vascular resistance in essential hypertension: duplex-Doppler ultrasonographic evaluation. Angiology 51(8):667–675
Mostbeck GH, Kain R, Mallek R, Derfler K, Walter R, Havelec L, Tscholakoff D (1991) Duplex Doppler sonography in renal parenchymal disease: histopathologic correlation. J Ultrasound Med 10(4):189–194
Tublin ME, Bude RO, Platt JF (2003) Review. The resistive index in renal Doppler sonography: where do we stand? AJR 180(4):885–892
Nichols WW (2005) Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 18(1 Pt 2):3S–10S
Leite-Moreira AF, Correia-Pinto J, Gillebert TC (1999) Afterload induced changes in myocardial relaxation: a mechanism for diastolic dysfunction. Cardiovasc Res 43(2):344–353
MacIsaac RJ, Thomas MC, Panagiotopoulos S, Smith TJ, Hao H, Matthews DG, Jerums G, Burrell LM, Srivastava PM (2008) Association between intrarenal arterial resistance and diastolic dysfunction in type 2 diabetes. Cardiovasc Diabetol 7:15
Ohuchi H, Ikado H, Noritake K, Miyazaki A, Yasuda K, Yamada O (2013) Impact of central venous pressure on cardiorenal interactions in adult patients with congenital heart disease after biventricular repair. Congenit Heart Dis 8(2):103–110
Ciccone MM, Iacoviello M, Gesualdo L, Puzzovivo A, Antoncecchi V, Doronzo A, Monitillo F, Citarelli G, Paradies V, Favale S (2014) The renal arterial resistance index: a marker of renal function with an independent and incremental role in predicting heart failure progression. Eur J Heart Fail 16(2):210–216
Mule G, Geraci G, Geraci C, Morreale M, Cottone S (2015) The renal resistive index: is it a misnomer? Intern Emerg Med 10(8):889–891
Geraci G, Mule G, Costanza G, Mogavero M, Geraci C, Cottone S (2016) Relationship between carotid atherosclerosis and pulse pressure with renal hemodynamics in hypertensive patients. Am J Hypertens 29(4):519–527
Alehagen U, Benson L, Edner M, Dahlstrom U, Lund LH (2015) Association between use of statins and mortality in patients with heart failure and ejection fraction of >/=50. Circ Heart Fail 8(5):862–870
Fukuta H, Goto T, Wakami K, Ohte N (2016) The effect of statins on mortality in heart failure with preserved ejection fraction: a meta-analysis of propensity score analyses. Int J Cardiol 214:301–306
Acknowledgements
The authors wish to thank all of the patients who provided their consent to participate. We thank Dr. Masaaki Izumi for technical assistance, and Ms. Wendy Alexander-Adams and Mr. John Martin for their encouragement and assistance with the reporting of our findings in English.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Okumura has received research funding from Bayer Healthcare, Daiichi-Sankyo, Bristol-Meyers Squibb, Nippon Boehringer Ingelheim, Pfizer, TORAY, and Boston Scientific Japan and has accepted remuneration from Bayer Healthcare, Daiichi-Sankyo, and Bristol-Meyers Squibb. T. Nakai is affiliated with the Department of Medicine, Division of Advanced Therapeutics for Cardiac Arrhythmias established using funds from Abbott Medical, Biotronik Japan, Medtronic Japan, Japan Lifeline; and Boston Scientific Japan. T. Nakai received lecture fees from Abbott Medical, Biotronik Japan, and Medtronic Japan. All of the other authors have no conflict of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aizawa, Y., Okumura, Y., Saito, Y. et al. Association of renal resistance index and arterial stiffness on clinical outcomes in patients with mild-to-moderate renal dysfunction and presence or absence of heart failure with preserved ejection fraction. Heart Vessels 35, 1699–1708 (2020). https://doi.org/10.1007/s00380-020-01649-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-020-01649-2