Abstract
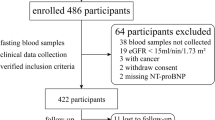
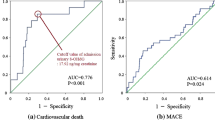
Hyperuricemia is known to be associated with adverse outcomes in cardiovascular intensive care patients, but its mechanisms are unknown. A total of 569 emergency department patients were prospectively analyzed and assigned to intensive care (ICU group, n = 431) or other departments (n = 138). Uric acid (UA) levels were significantly higher in the intensive care patients (6.3 [5.1–7.6] mg/dl vs. 5.8 [4.6–6.8] mg/dL). The plasma xanthine oxidoreductase (XOR) activity in the ICU group (68.3 [21.2–359.5] pmol/h/mL) was also significantly higher than that in other departments (37.2 [15.1–93.6] pmol/h/mL). Intensive care patients were divided into three groups according to plasma XOR quartiles (Q1, low-XOR, Q2/Q3, normal-XOR, and Q4, high-XOR group). A multivariate logistic regression model showed that lactate (per 1.0 mmol/L increase, OR 1.326; 95%, CI 1.166–1.508, p < 0.001) and the Acute Physiology and Chronic Health Evaluation II score (per 1.0 point increase, OR 1.095, 95% CI 1.034–1.160, p = 0.002) were independently associated with the high-XOR group. In-hospital mortality was significantly higher in the high-XOR group (n = 28, 26.2%) than in the normal- (n = 11, 5.1%) and low- (n = 9, 8.3%) XOR groups. The high-XOR group (vs. normal-XOR group) was independently associated with the in-hospital mortality (OR 2.934; 95% CI 1.170–7.358; p = 0.022). Serum UA levels and plasma XOR activity were high in patients admitted to intensive care. The enhanced XOR activity may be one of the mechanisms under which hyperuricemia was associated with adverse outcomes in patients requiring cardiovascular intensive care.




Similar content being viewed by others
Availability of data and materials section
The dataset supporting the conclusions of this article is included within the article.
References
Okazaki H, Shirakabe A, Kobayashi N, Hata N, Shinada T, Matsushita M, Yamamoto Y, Shibuya J, Shiomura R, Nishigoori S, Asai K, Shimizu W (2016) The prognostic impact of uric acid in patients with severely decompensated acute heart failure. J Cardiol 68:384–391
Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ (2003) Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 107:1991–1997
Kobayashi N, Hata N, Tsurumi M, Shibata Y, Okazaki H, Shirakabe A, Takano M, Seino Y, Shimizu W (2018) Relation of coronary culprit lesion morphology determined by optical coherence tomography and cardiac outcomes to serum uric acid levels in patients with acute coronary syndrome. Am J Cardiol 122:17–25
Pagidipati NJ, Hess CN, Clare RM, Akerblom A, Tricoci P, Wojdyla D, Keenan RT, James S, Held C, Mahaffey KW, Klein AB, Wallentin L, Roe MT (2017) An examination of the relationship between serum uric acid level, a clinical history of gout, and cardiovascular outcomes among patients with acute coronary syndrome. Am Heart J 187:53–61
Pehlivanlar-Kucuk M, Kucuk AO, Ozturk CE, Er MC, Ulger F (2018) The association between serum uric acid level and prognosis in critically ill patients, uric acid as a prognosis predictor. Clin Lab 64:1491–1500
Shibata Y, Shirakabe A, Okazaki H, Matsushita M, Sawatani T, Uchiyama S, Tani K, Kobayashi N, Otsuka T, Hata N, Asai K, Shimizu W (2019) The prognostic impact of the uric acid level in patients who require cardiovascular intensive care - is serum uric acid a surrogate biomarker for critical patients in the non-surgical intensive care unit? EHJ Acute Cardovasc Care. https://doi.org/10.1177/2048872618822473
Agarwal A, Banerjee A, Banerjee UC (2011) Xanthine oxidoreductase: a journey from purine metabolism to cardiovascular excitation-contraction coupling. Crit Rev Biotechnol 31:264–280
Robert AM, Robert L (2014) Xanthine oxido-reductase, free radicals and cardiovascular disease. A critical review. Pathol Oncol Res 20:1–10
Otaki Y, Watanabe T, Kinoshita D, Yokoyama M, Takahashi T, Toshima T, Sugai T, Murase T, Nakamura T, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Miyamoto T, Kubota I (2017) Association of plasma xanthine oxidoreductase activity with severity and clinical outcome in patients with chronic heart failure. Int J Cardiol 228:151–157
Okazaki H, Shirakabe A, Matsushita M, Shibata Y, Sawatani T, Uchiyama S, Tani K, Murase T, Nakamura T, Takayasu T, Asano M, Kobayashi N, Hata N, Asai K, Shimizu W (2019) Plasma xanthine oxidoreductase activity in patients with decompensated acute heart failure requiring intensive care. ESC Heart Failure 6:336–343
Nakatani A, Nakatani S, Ishimura E, Murase T, Nakamura T, Sakura M, Tateishi Y, Tsuda A, Kurajoh M, Mori K, Emoto M, Inaba M (2017) Xanthine oxidoreductase activity is associated with serum uric acid and glycemic control in hemodialysis patients. Sci Rep 7:15416
Washio KW, Kusunoki Y, Murase T, Nakamura T, Osugi K, Ohigashi M, Sukenaga T, Ochi F, Matsuo T, Katsuno T, Moriwaki Y, Yamamoto T, Namba M, Koyama H (2017) Xanthine oxidoreductase activity is correlated with insulin resistance and subclinical inflammation in young humans. Metabolism 70:51–56
Furuhashi M, Matsumoto M, Tanaka M, Moniwa N, Murase T, Nakamura T, Ohnishi H, Saitoh S, Shimamoto K, Miura T (2018) Plasma xanthine oxidoreductase activity as a novel biomarker of metabolic disorders in a general population. Circ J 82:1892–1899
Fujimura Y, Yamauchi Y, Murase T, Nakamura T, Fujita SI, Fujisaka T, Ito T, Sohmiya K, Hoshiga M, Ishizaka N (2017) Relationship between plasma xanthine oxidoreductase activity and left ventricular ejection fraction and hypertrophy among cardiac patients. PLoS ONE 12:e0182699
Murase T, Nampei M, Oka M, Miyachi A, Nakamura T (2016) A highly sensitive assay of human plasma xanthine oxidoreductase activity using stable isotope-labeled xanthine and LC/TQMS. J Chromatogr B Analyt Technol Biomed Life Sci 1039:51–58
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Critical Care Med 13:818–829
Murase T, Nampei M, Oka M, Ashizawa N, Matsumoto K, Miyachi A, Nakamura T (2016) Xanthine oxidoreductase activity assay in tissues using stable isotope-labeled substrate and liquid chromatography high-resolution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 1008:189–197
Murase T, Oka M, Nampei M, Miyachi A, Nakamura T (2016) A highly sensitive assay for xanthine oxidoreductase activity using a combination of [(13) C2, (15) N2 ]xanthine and liquid chromatography/triple quadrupole mass spectrometry. J Labelled Comp Radiopharm 59:214–220
Watanabe K, Shishido T, Otaki Y, Watanabe T, Sugai T, Toshima T, Takahashi T, Yokoyama M, Kinoshita D, Murase T, Nakamura T, Wanezaki M, Tamura H, Nishiyama S, Takahashi H, Arimoto T, Yamauchi S, Yamanaka T, Miyamoto T, Kubota I, Watanabe M (2018) Increased plasma xanthine oxidoreductase activity deteriorates coronary artery spasm. Heart Vessels 34:1–8
Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, Harrison DG (2003) Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation 107:1383–1389
Ali OS, Abdelgawad HM, Mohammed MS, El-Awady RR (2014) Ischemic heart diseases in Egypt: role of xanthine oxidase system and ischemia-modified albumin. Heart Vessels 29:629–637
Kurajoh M, Fukumoto S, Emoto M, Murase T, Nakamura T, Ishihara T, Go H, Yamamoto K, Nakatani S, Tsuda A, Yamada S, Morioka T, Mori K, Imanishi Y, Inaba M (2019) Independent association of plasma xanthine oxidoreductase activity with serum uric acid level based on stable isotope-labeled xanthine and liquid chromatography/triple quadrupole mass spectrometry: MedCity21 health examination registry. Clin Chem Lab Med. https://doi.org/10.1515/cclm-2019-0199
Pacher P, Nivorozhkin A, Szabo C (2006) Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 58:87–114
Narsale AA, Enos RT, Puppa MJ, Chatterjee S, Murphy EA, Fayad R, Pena MO, Durstine JL, Carson JA (2015) Liver inflammation and metabolic signaling in ApcMin/+ mice: the role of cachexia progression. PLoS ONE 10:e0119888
Kelley EE (2015) Dispelling dogma and misconceptions regarding the most pharmacologically targetable source of reactive species in inflammatory disease, xanthine oxidoreductase. Arch Toxicol 89:1193–1207
Acknowledgements
We are grateful to the staff of the ICU and medical records office at Chiba Hokusoh Hospital, Nippon Medical School, Japan, for collecting the medical data.
Funding
The authors declare that they have no competing sources of funding.
Author information
Authors and Affiliations
Contributions
YS AS participated in the conception and design; data collection, analysis, and interpretation; statistical analysis; and drafting of the manuscript. TM and TN measured the XOR activity. YS, AS, HO, MM, HG, SS, KK, KK, KT, NK, NH, KA and WS participated in the conception and design and data collection. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The research ethics committee of Nippon Medical School Chiba Hokusoh Hospital approved the study protocol.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shibata, Y., Shirakabe, A., Okazaki, H. et al. Plasma xanthine oxidoreductase (XOR) activity in patients who require cardiovascular intensive care. Heart Vessels 35, 1390–1400 (2020). https://doi.org/10.1007/s00380-020-01608-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-020-01608-x