Abstract
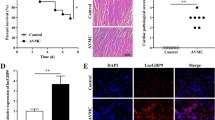
Viral myocarditis (VMC) is a widely studied but poorly understood inflammatory cardiomyopathy which mainly affects children and young adults and results in adverse outcomes. Cardiomyocyte apoptosis was reported important in the progress of coxsackievirus B3 (CVB3)-induced VMC and the blocking of this process may contribute to the therapeutic effect towards VMC. Therefore, this study was designed to explore whether survivin, one of the strongest antiapoptotic proteins, can protect cardiomyocytes from apoptosis in VMC and to discover its related mechanisms. Here, the cultured neonatal mouse cardiomyocytes (NMCs) were exposed to CVB3 to establish the cell model of VMC and the results of Western Blot showed that the protein expression of survivin in CVB3-infected NMCs varied at different post-infection time. Lentivirus was next used to examine the function of survivin in CVB3-infected NMCs. TUNEL assay demonstrated that the overexpression of survivin interrupted CVB3-induced apoptosis. It was next examined whether caspase-3 and -9 were involved in the antiapoptotic pathway initiated by survivin via Western Blot. The results showed a reverse relationship between the protein expression of survivin and that of cleaved caspase-3 and cleaved caspase-9, suggesting that survivin may attenuate apoptosis through restraining the activity of caspase-3 and -9. Moreover, the supernatant fluid of cultured NMCs was extracted to detect the quantitation of released lactate dehydrogenase (LDH) and a sharp decrease was discovered in the survivin-overexpressed group compared to the CVB3-infected group, indicating a protective role of survivin in the cell model of CVB3-induced myocarditis. This study demonstrated that survivin was triggered by CVB3 infection in NMCs and survivin executed its antiapoptotic effects via caspase-3- and caspase-9-dependent signaling pathway.






Similar content being viewed by others
References
Esfandiarei M, McManus BM (2008) Molecular biology and pathogenesis of viral myocarditis. Ann Rev Pathol 3:127–155
Global Burden of Disease Study (2013) Collaborators (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386(9995):743–800
Pollack A, Kontorovich AR, Fuster V, Dec GW (2015) Viral myocarditis–diagnosis, treatment options, and current controversies. Nat Rev Cardiol 12(11):670–680
Huang TF, Wu XH, Wang X, Lu IJ (2016) Fas-FasL expression and myocardial cell apoptosis in patients with viral myocarditis. Genet Mol Res 15(2):7607
Martin U, Nestler M, Munder T, Zell R, Sigusch HH, Henke A (2004) Characterization of coxsackievirus B3-caused apoptosis under in vitro conditions. Med Microbiol Immunol 193(2–3):133–139
Henke A, Launhardt H, Klement K, Stelzner A, Zell R, Munder T (2000) Apoptosis in coxsackievirus B3-caused diseases: interaction between the capsid protein VP2 and the proapoptotic protein siva. J Virol 74(9):4284–4290
Colston JT, Chandrasekar B, Freeman GL (1998) Expression of apoptosis-related proteins in experimental coxsackievirus myocarditis. Cardiovasc 38(1):158–168
Kytö V, Saraste A, Fohlman J, Ilbäck NG, Harvala H, Vuorinen T, Hyypiä T (2002) Cardiomyocyte apoptosis after antiviral WIN 54954 treatment in murine coxsackievirus B3 myocarditis. Scand Cardiovasc J 36(3):187–192
Huber SA (2000) T cells expressing the gamma delta T cell receptor induce apoptosis in cardiac myocytes. Cardiovasc Res 45(3):579–587
Kytö V, Saraste A, Saukko P, Henn V, Pulkki K, Vuorinen T, Voipio-Pulkki LM (2004) Apoptotic cardiomyocyte death in fatal myocarditis. Am J Cardiol 94(6):746–750
Sah NK, Khan Z, Khan GJ, Bisen PS (2006) Structural, functional and therapeutic biology of survivin. Cancer Lett 244(2):164–171
Gravina G, Wasén C, Garcia-Bonete MJ, Turkkila M, Erlandsson MC, Töyrä Silfverswärd S, Brisslert M, Pullerits R, Andersson KM, Katona G, Bokarewa MI (2007) Survivin in autoimmune diseases. Autoimmun Rev 16(8):845–855
Yang M, Li B, Liu J, Sun H (2015) Protection effect of survivin protein overexpression on acute myocardial infarction in rats. Int J Clin Exp Med 8(8):12995–13000
Blum R, Jacob-Hirsch J, Rechavi G, Kloog Y (2006) Suppression of survivin expression in glioblastoma cells by the Ras inhibitor farnesylthiosalicylic acid promotes caspase-dependent apoptosis. Mol Cancer Ther 5(9):2337–2347
Chandele A, Prasad V, Jagtap JC, Shukla R, Shastry PR (2004) Upregulation of survivin in G2/M cells and inhibition of caspase 9 activity enhances resistance in staurosporine-induced apoptosis. Neoplasia 6(1):29–40
Lossi L, Cocito C, Alasia S, Merighi A (2016) Ex vivo imaging of active caspase 3 by a FRET-based molecular probe demonstrates the cellular dynamics and localization of the protease in cerebellar granule cells and its regulation by the apoptosis-inhibiting protein survivin. Mol Neurodegener 11:34
Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH (2001) An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 40(4):1117–1123
Ehler E, Moore-Morris T, Lange S (2013) Isolation and culture of neonatal mouse cardiomyocytes. J Vis Exp 79:50154
Li WL, Lee MR, Cho MY (2016) The small molecule survivin inhibitor YM155 may be an effective treatment modality for colon cancer through increasing apoptosis. Biochem Biophys Res Commun 471(2):309–314
Sam MR, Ghoreishi S (2018) Prodigiosin produced by Serratia marcescens inhibits expression of MMP-9 and survivin and promotes caspase-3 activation with induction of apoptosis in acute lymphoblastic leukaemia cells. J Appl Microbiol 125(4):1017–1029
Yenkejeh RA, Sam MR, Esmaeillou M (2017) Targeting survivin with prodigiosin isolated from cell wall of Serratia marcescens induces apoptosis in hepatocellular carcinoma cells. Hum Exp Toxicol 36(4):402–411
Zwerts F, Lupu F, De Vriese A, Pollefeyt S, Moons L, Altura RA, Jiang Y, Maxwell PH, Hill P, Oh H, Rieker C, Collen D, Conway SJ, Conway EM (2007) Lack of endothelial cell survivin causes embryonic defects in angiogenesis, cardiogenesis, and neural tube closure. Blood 109(11):4742–4752
Gregory CD, Paterson M (2018) An apoptosis-driven ‘onco-regenerative niche’: roles of tumour-associated macrophages and extracellular vesicles. Philos Trans R Soc Lond B Biol Sci 373(1737):20170003
Shim JM, Kim J, Tenson T, Min J-Y, Kainov DE (2017) Influenza virus infection, interferon response, viral counter-response, and apoptosis. Viruses 9(8):223
Zhou X, Jiang W, Liu Z, Liu S, Liang X (2017) Virus infection and death receptor-mediated apoptosis. Viruses 9(11):316
Abbate A, Sinagra G, Bussani R, Hoke NN, Merlo M, Varma A, Toldo S, Salloum FN, Biondi-Zoccai GG, Vetrovec GW, Crea F, Silvestri F, Baldi A (2009) Apoptosis in patients with acute myocarditis. Am J Cardiol 104(7):995–1000
Fuse K, Kodama M, Okura Y, Ito M, Hirono S, Kato K, Hanawa H, Aizawa Y (2000) Predictors of disease course in patients with acute myocarditis. Circulation 102(23):2829–2835
Saraste A, Arola A, Vuorinen T, Kytö V, Kallajoki M, Pulkki K, Voipio-Pulkki LM, Hyypiä T (2003) Cardiomyocyte apoptosis in experimental coxsackievirus B3 myocarditis. Cardiovasc Pathol 12(5):255–262
Zorc-Pleskovic R, Alibegović A, Zorc M, Milutinović A, Radovanović N, Petrović D (2006) Apoptosis of cardiomyocytes in myocarditis. Folia Biol (Praha) 52(1–2):6–9
Kang PM, Izumo S (2000) Apoptosis and heart failure: a critical review of the literature. Circ Res 86(11):1107–1113
Fogarty CE, Bergmann A (2017) Killers creating new life: caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ 24(8):1390–1400
Bardak H, Uğuz AC, Bardak Y (2017) Curcumin regulates intracellular calcium release and inhibits oxidative stress parameters, VEGF, and caspase-3/-9 levels in human retinal pigment epithelium cells. Physiol Int 104(4):301–315
Kim H, Choi H, Lee SK (2016) Epstein-Barr virus microRNA miR-BART20-5p suppresses lytic induction by inhibiting BAD-mediated caspase-3-dependent apoptosis. J Virol 90(3):1359–1368
Ying X, Peng Y, Zhang J, Wang X, Wu N, Zeng Y, Wang Y (2014) Endogenous alpha-crystallin inhibits expression of caspase-3 induced by hypoxia in retinal neurons. Life Sci 111(1–2):42–46
Sam MR, Tavakoli-Mehr M, Safaralizadeh R (2018) Omega-3 fatty acid DHA modulates p53, survivin, and microRNA-16-1 expression in KRAS-mutant colorectal cancer stem-like cells. Genes Nutr 13:8
Chantalat L, Skoufias DA, Kleman JP, Jung B, Dideberg O, Margolis RL (2000) Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alpha-helical extensions. Mol Cell 6(1):183–189
Verdecia MA, Huang H, Dutil E, Kaiser DA, Hunter T, Noel JP (2000) Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat Struct Biol 7(7):602–608
Eckelman BP, Salvesen GS, Scott FL (2006) Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep 7(10):988–994
Marusawa H, Matsuzawa S, Welsh K, Zou H, Armstrong R, Tamm I, Reed JC (2003) HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO J 22(11):2729–2740
O'Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Altieri DC (2000) Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA 97(24):13103–13107
Ursu ON, Sauter M, Ettischer N, Kandolf R, Klingel K (2014) Heme oxygenase-1 mediates oxidative stress and apoptosis in coxsackievirus B3-induced myocarditis. Cell Physiol Biochem 33(1):52–66
Acknowledgements
This study was funded by the National Nature Science Foundation of China (Grant no. 81870275 and 81900346, China).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We do not have conflict of interests about the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, R., Wu, T., Li, P. et al. The protection effects of survivin in the cell model of CVB3-induced viral myocarditis. Heart Vessels 35, 1171–1179 (2020). https://doi.org/10.1007/s00380-020-01607-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-020-01607-y