Abstract
This multi-center prospective non-randomized comparative study investigated the effects of pitavastatin in patients with peripheral artery disease (PAD) in terms of exercise tolerance capacities and peripheral CD34+/133+ cell numbers. At baseline, a peripheral blood test was administered to 75 patients with PAD, along with a treadmill exercise test using the Skinner–Gardner protocol to measure asymptomatic walking distance (AWD) and maximum walking distance (MWD). Each patient was assigned to a 6-month pitavastatin treatment group (n = 53) or a control group (n = 22), according to the patient’s preference. The tests were repeated in both groups at 3 and 6 months. Baseline AWD and MWD correlated positively with the ankle-brachial pressure index (r = 0.342, p = 0.0032 and r = 0.324, p = 0.0054, respectively). Both AWD and MWD values improved at 3 and 6 months compared with baseline, and the degrees of their improvement were higher in the pitavastatin treatment group. CD34+/133+ cell numbers did not change over time or between groups. Eighty-seven percent of patients in the treatment group attained low-density lipoprotein cholesterol levels below 100 mg/dL after 3 months. The study shows that pitavastatin may be effective in increasing exercise tolerance capacity in patients with PAD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral artery disease (PAD), the prevalence of which increases with advancing age and reaches approximately 6% in late sixties, occasionally results in poor or even fatal clinical outcomes because of concomitant coronary artery disease (CAD) or cerebrovascular disease [1, 2]. Critical limb ischemia, often leading to lower limb amputation, compromises activities of daily living and lowers overall life prognosis [3, 4]. According to the Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) [5], exercise prior to revascularization is recommended in patients with non-critical limb ischemia PAD. For pharmacotherapy, cilostazol is recommended as Class I agent [5,6,7]. Statins were previously recommended as Class IIa agents [5, 6]; however, the most recent American Heart Association/American College of Cardiology (AHA/ACC) guideline recommends the use of statins as Class I agents, because statin therapy improves both cardiovascular and limb outcomes in patients with PAD [7]. TASC II and AHA/ACC guidelines recommend that low-density lipoprotein cholesterol (LDL-C) levels in patients with PAD not exceed 100 mg/dL [4, 5, 7]. Statins have been reported to be effective in primary and secondary prevention of coronary arterial events by lowering lipids and exerting various pleiotropic effects, such as improvement of endothelial function and neovascularization [8] following the increase in number and function of endothelial progenitor cells (EPCs) [9, 10], improvement of arterial endothelial function [11,12,13,14], a decrease in oxidative stress [14, 15], and regression of plaque volume by non-steroidal isoprenoid products [8, 16,17,18,19]. Sata et al. [8] demonstrated that pitavastatin, cerivastatin, and fluvastatin inhibited atherosclerotic lesion progression in apolipoprotein E-deficient mice, while augmenting blood flow recovery and capillary formation in ischemic hind limbs. These results suggest that statins may not promote the development of cancer and atherosclerosis at doses that augment collateral flow growth in ischemic tissues. Furthermore, an increase in EPC numbers by exercise can lead to angiogenesis or neovascularization [20,21,22]. Despite the TASC-II recommendation for statin therapy in patients with PAD, few studies on using statins in these patients have been reported, and the body of evidence backing this recommendation is not robust [23,24,25]. Thus, we characterized the effects of pitavastatin on exercise tolerance capacity and the numbers of peripheral CD34+/133+ progenitor cells in patients with PAD.
Methods
Study patients
Inclusion criteria were patients with PAD; age from 45 to 80; more than 3 months after initiation of antiplatelet agents; LDL-C levels: 100–160 mg/dL, and no statin therapy. Patients with PAD were selected from the outpatient clinic chart in each participating institution. All patients demonstrated either ankle-brachial pressure index (ABI) below 0.90 or significant organic stenosis (≥75% diameter) in iliac, femoral, or popliteal arteries using arteriography, computed tomography, Doppler ultrasonography, or magnetic resonance. Exclusion criteria included PAD with Fontaine stage IV; unstable angina; left ventricular ejection fraction <50%; current smoking habit; difficulty participating in the exercise test owing to neurological, ophthalmological, or orthopedic dysfunction; having more than one acute myocardial infarction, cerebrovascular event, peripheral artery revascularization by catheter angioplasty, or bypass grafting within the preceding 3 months; taking LDL apheresis. We recruited 75 patients with PAD.
Ankle-brachial pressure index (ABI)
The ABI is a rapid, non-invasive, and reliable measurement method that detects and quantifies PAD [26]. The sensitivity of the ABI to detect PAD has been reported in clinical trials to be approximately 95%, with a specificity near 100% [26, 27]. ABI was measured in each subject after a 5-min rest period in the supine position. A cuff was attached to the brachial artery to measure systolic blood pressure. Another cuff was placed around the ankle. The blood pressure cuff was inflated to 20 mmHg above the systolic blood pressure and deflated over the artery in 2-mm/s increments. The ABI was calculated in each leg as the ratio of ipsilateral ankle systolic pressure to the higher of two systolic brachial pressures.
Testing assay
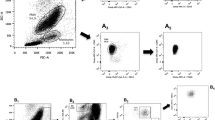
At baseline and at 3 and 6 months, fasting blood samples were taken from the cubital vein at rest just prior to the treadmill exercise test. We measured levels of total cholesterol (T-Chol), triglycerides (TG), LDL-C, high-density lipoprotein cholesterol (HDL-C), creatine kinase, plasma glucose, glycohemoglobin A1c (HbA1c), plasma insulin, high-sensitivity C-reactive protein (hsCRP), and the number of CD34+/133+ cells. Parameters of hsCRP and the number of CD34+/133+ cells were measured at one central location by SRL Co., and all other parameters in each patient’s healthcare institution. T-Chol, TG, HDL-C, LDL-C, and plasma glucose serum levels were measured by enzymatic methods, HbA1c by high-performance liquid chromatography, plasma insulin levels by enzyme immunoassay, and hsCRP by a monoclonal antibody employing latex that allows measurement of samples with low (0.01 mg/dL) to high (42 mg/dL) concentrations (Nanopia CRP; Daiichi Pure Chemicals, Tokyo, Japan). The numbers of CD34+/45+/133+ cells were measured by trained technicians using fluorescence-activated cell sorting (FACS) (FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA) with anti-human CD34+, CD45+, and CD133+ antibodies (BD Biosciences) as described elsewhere [21, 28]. After gating CD45+ cells from whole blood, the numbers of cells double-positive for CD34 and CD133 were measured [21, 28].
Definition of risk factors
Risk factors were identified from the medical history or hospital data and included HbA1c levels ≥6.5% for diabetes mellitus (DM), T-Chol levels ≥220 mg/dL and/or LDL-C levels ≥140 mg/dL for hypercholesterolemia, systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg for hypertension, and body mass index ≥25 for obesity, as defined by the Japan Society for the Study of Obesity.
Evaluation of exercise tolerance capacity
At baseline and at 3 and 6 months, the patients underwent a symptom-limited treadmill exercise stress test following the Skinner–Gardner protocol, which consists of a progressive graded workload with a constant speed of 2 miles/h (3.2 km/h) and a 2% increase in grade every 2 min from 0 to 12% [29]. During the treadmill exercise test, standardized verbal encouragement was given, and patients were continuously monitored for hemodynamic response (heart rate, heart rhythm, and blood pressure) to exercise until they experienced leg pain, or until the appearance of angina symptoms. Asymptomatic walking distance (AWD) was calculated as the distance in meters during the treadmill test at the onset of claudication, regardless of whether this symptom manifested as muscle pain, aches, cramps, numbness, or fatigue. Maximum walking distance (MWD) was calculated as the distance walked during the treadmill test before stopping due to claudication.
Study protocol
The study was a prospective non-randomized comparative multicenter study comparing two PAD groups with or without pitavastatin treatment. Eight healthcare facilities in Japan participated in this study. This protocol was approved by the Ethics Committee of each institution, and written informed consent was obtained from all patients in accordance with the ethical standards of the institutional and the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Fasting blood and physiological tests were administered between 9:00 AM and 11:00 AM at each outpatient department. At baseline, blood and urine samples were collected from all patients, the ABI was calculated, and the treadmill test was conducted to evaluate each patient’s exercise tolerance capacity, AWD, and MWD [29]. Peripheral blood parameters included blood chemistry, lipid profiles, and CD34+/133+ cell numbers as a marker for endothelial progenitor cells, the measurement of which has been described elsewhere [21, 28]. After giving general information about PAD to each participant and taking baseline laboratory and physiological measurements, all patients were divided into two groups according to their individual choices: A 6-month pitavastatin treatment group (P, n = 53), and a control group that did not receive any statins (C, n = 22). The daily dose of pitavastatin was prescribed by each patient’s physician according to the TASC-II guideline (LDL-C below 100 mg/dL) [5]. Following group assignment, the tests were repeated in both groups at the 3- and 6-month marks. During the study period, all patients were advised to maintain a consistent frequency and intensity of daily exercise as far as possible, and to record their daily walking time and pedometer counts in an exercise journal [30].
Statistical analysis
Data were analyzed using JMP 7.01J software (SAS Institute, Cary, NC, USA). Continuous variables are expressed as mean ± standard deviation (SD). Mean values were compared between two groups, where appropriate, by Student’s t test or two-way analysis of variance followed by the Tukey–Kramer post hoc test. Categorical variables are expressed as frequencies and were compared using Chi-squared analysis. Correlations were assessed using Fisher’s coefficient (r). Probability values of p < 0.05 were considered statistically significant.
Results
During the study period, three patients withdrew because of major adverse events in the control group: Subarachnoid hemorrhage (n = 1), brain stem cerebral embolism (n = 1), and intestinal bleeding due to rectal cancer (n = 1). The other 72 patients completed the study protocol without any cardiovascular events. In the statin treatment group, no adverse events occurred during the study. In the control group, medication regimens were not altered during the study period. Baseline patient characteristics are shown in Table 1. T-Chol, HDL-C, and LDL-C levels were similar between the two groups, but HbA1c and TG levels, as well as percentiles of ischemic heart disease and diabetes, were higher in the statin group than in the control group.
Table 2 displays the serial changes in blood parameters. AWD and MWD values for the baseline treadmill exercise test correlated with ABI (AWD: r = 0.342, p = 0.0032; MWD: r = 0.324, p = 0.0054; Fig. 1). Daily doses of pitavastatin ranged as follows: Four milligrams (6% of patients), 2 mg (60% of patients), 1 mg (30% of patients), and 0.5 mg (4% of patients). At the 6-month mark, lipid parameters improved in the statin group, and 87% of patients in the group achieved the recommended TASC-II guideline (LDL-C below 100 mg/dL). At 3 and 6 months, both AWD and MWD in the statin group improved compared with the group’s baseline values (AWD: p = 0.0013; MWD: p = 0.0004). AWD and MWD values in the control group did not change significantly over time (Fig. 2). At 6 months, both AWD and absolute change in AWD were greater in the statin group than in the control group, and MWD in the statin group was generally greater than in the control group (Fig. 3).
Changes in exercise tolerance capacity in the treadmill exercise test at 3 and 6 months. Serial changes in asymptomatic walking distance (a) and maximum walking distance (b), and absolute changes in asymptomatic walking distance (c) and maximum walking distance (d) from baseline. 3 m 3 months, 6 m 6 months, C control group, m meters, P pitavastatin treatment group
Serial changes in exercise tolerance capacity parameters between baseline and the 6-month mark. Absolute changes in asymptomatic walking distance (AWD) and maximum walking distance (MWD) compared with baseline at 6 months. ABI ankle-brachial pressure index, C control group, P pitavastatin treatment group
Furthermore, in patients with ABI below 0.7, the degrees of improvement in exercise tolerance capacity in the statin group were significantly greater than those in the control group (Fig. 4). hsCRP as a marker for inflammation decreased after pitavastatin treatment, especially in patients with diabetes. The number of CD34+/133+ cells did not change significantly in either group, and the two groups did not differ (Table 2; Fig. 5).
Serial changes in peripheral Log10CD34+/133+ cell numbers following a 6-month treatment. a Comparison of the pitavastatin treatment group (P) and the control group (C). b Comparison between groups by treatment and ankle-brachial pressure index (ABI). c Comparison between groups by treatment and Log10CD34+/133+ cell numbers
Discussion
In the present study, we defined exercise capacity by the AWD and MWD at baseline, and found that both values were greater in the statin group after 6 months, and were accompanied by effective lipid lowering. The numbers of CD34+/133+ cells did not change significantly over time and did not differ between groups.
Baseline correlation of ABI with exercise tolerance capacity
In the baseline treadmill test, exercise tolerance capacities, measured as AWD and MWD, were positively correlated with lower baseline ABI values. This finding may be leveraged to estimate the approximate length and intensity of daily exercise as therapy.
Changes in exercise tolerance capacities after a 6-month pitavastatin treatment
In the pitavastatin treatment group, the two exercise capacity parameters, AWD and MWD, improved over time, whereas those in the control group did not change significantly. The absolute change in AWD at 6 months was greater in the statin group than in control the control group. Exercise improves walking performance in patients with PAD [4, 5, 30]. The effect of statins on walking performance in patients with PAD varies in published studies, but most researchers concurred that statins improve walking performance [23, 24]. Our findings concur with those reported by Mohler et al., who used atorvastatin to improve walking distance in patients with PAD [24].
Furthermore, in patients with ABI below 0.7 (32 of 75 patients), the absolute changes in AWD and MWD in the statin group were significantly greater than those in the control group. Although the sample size was small, this finding may suggest the greater efficacy of statins in patients with PAD with moderate to severe arterial stenosis than in those with only mild stenosis. The present study indicates that statins may be more effective in patients with low ABI values in terms of exercise tolerance capacity. Statins exert pleiotropic effects such as plaque stability, plaque volume regression, and anti-inflammatory action [8, 16,17,18,19, 31]. One possible explanation is the plaque volume regression at the culprit lesion. In the pitavastatin group, ABI values below 0.7 increased after a 6-month statin treatment (p = 0.0004, data not shown), while no significant change was observed in patients with ABI over 0.7 regardless of changes in LDL-C, HDL-C, and hsCRP. Lesions with lower ABI exert higher plaque burdens, and plaque regression and stability by statin treatment may lead to improvement of ABI and exercise tolerance capacity.
CD34+/133+ cell numbers in peripheral blood following statin treatment
In contrast with our expectations, the number of CD34+/133+ cells did not increase significantly after a 6-month statin treatment. According to previous reports, exercise [9, 10, 21, 32] or statin treatment [23, 24] can increase the numbers of peripheral EPCs, while diabetes can decrease them [33]. Exercise increases the number of EPCs because increased vascular shear stress boosts the production of nitric oxide [9, 10, 21]. Statin treatment can induce the movement of EPCs from bone marrow to peripheral blood [23, 24] and augment collateral flow growth in ischemic tissues [8]. It is possible that our sample size was too small to detect differences. In addition, patients presented with individual effect modifiers, such as advancing age, DM, and varying levels of daily activity; all these factors may have influenced the number of CD34+/133+ cells. A randomized study with a much larger sample size would therefore be required.
Risk factors and PAD
As shown in Table 1, most patients had coronary risk factors, and more than half (40 of 75) of these patients with PAD also had CAD, a percentage similar to that in previous reports [5, 23, 24]. Statins have been reported to assist in lipid management in patients with CAD and PAD [16,17,18,19, 31, 34]. Though each patient in our study made an individual decision regarding statin treatment after receiving a general explanation on lipid management, our numbers may reflect the baseline differences in TGs and percentage of concomitant CAD between the two groups. Thus, the patient background could not be equalized between the statin and control groups.
Achievement of LDL-C targets after a 6-month pitavastatin treatment
Eighty-five percent of patients with PAD treated with pitavastatin for 6 months achieved the recommended serum LDL-C target (under 100 mg/dL) without any significant side effects [5]. Overall adherence to statin administration was high in all groups (>95%). This pilot study suggests that oral administration of pitavastatin for 6 months is feasible in terms of safety and tolerance. Additionally, the findings indicate that pitavastatin may offer a modest benefit for functional capacity, as determined by initial and absolute claudication distance and self-reported walking speed. Ninety percent of patients with PAD in the statin group took a 1- or 2-mg daily dose of pitavastatin, and the maximal dose was 4 mg; these doses are standard in Japanese patients with atherosclerosis. Furthermore, CAD comorbidity is typically found in approximately 50% of patients with PAD, and is also treated by controlling lipid levels [5]. Use of a strong statin may reduce the need for revascularization, but reductions in rates of amputation were not observed in the previous studies [25]. Moderate-dose statin therapy is safe, and the minor risks are greatly outweighed by the benefits.
Other improvements in blood parameters following pitavastatin treatment
We found that pitavastatin treatment showed a tendency toward a decrease in inflammation, as measured by levels of hsCRP, especially in patients with diabetes; this was not observed in the control group. The present results regarding hsCRP reduction by strong statins are in line with the previous reports [35,36,37].
Conclusions
This pilot study, although small, showed that a 6-month treatment with pitavastatin is feasible in terms of safety and efficacy in managing serum lipid levels and improving exercise tolerance capacity in patients with PAD.
Study limitations
This study was conducted as a pilot study prior to a randomized, double-blind cohort study. Baseline patient characteristics varied, because patients were assigned not randomly but according to their own preferences, leading to a possible selection bias. To confirm our findings, a larger patient cohort assigned randomly to treatment groups would be required. In addition, patients engaged in non-supervised daily exercise, and the intensity and duration of exercise varied by patient. Third, maximal exercise distance by the treadmill test was determined objectively rather than subjectively, because the test was terminated at the discretion of the patient. Finally, we evaluated EPCs in peripheral blood as the numbers of CD34+/133+ cells, although other indices for EPC can be used in clinical settings [22, 38].
References
Alahdab F, Wang AT, Elraiyah TA, Malgor RD, Rizvi AZ, Lane MA, Prokop LJ, Montori VM, Conte MS, Murad MH (2015) A systematic review for the screening for peripheral arterial disease in asymptomatic patients. J Vasc Surg 61(3 Suppl):42S–53S
Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D (1992) Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 326:381–386
Dormandy J, Mahir M, Ascady G, Balsano F, De-Leeuw P, Blombery P, Bousser MG, Clement D, Coffman J, Deutschinoff A (1989) Fate of the patient with chronic leg ischaemia. J Cardiovasc Surg (Torino) 30:50–57
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B, American Association for Vascular Surgery, Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease, American Association of Cardiovascular and Pulmonary Rehabilitation, National Heart, Lung, and Blood Institute, Society for Vascular Nursing, TransAtlantic Inter-Society Consensus, Vascular Disease Foundation (2006) ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 47:1239–1312
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group (2007) Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 45(Suppl S):S5–S67
Regensteiner J, Ware JJ, McCarthy W, Zhang P, Forbes W, Heckman J, Hiatt WR (2002) Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. J Am Geriatr Soc 50:1939–1946
Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME (2017) 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 69(11):e71–e126
Sata M, Nishimatsu H, Osuga J, Tanaka K, Ishizaka N, Ishibashi S, Hirata Y, Nagai R (2004) Statins augment collateral growth in response to ischemia but they do not promote cancer and atherosclerosis. Hypertension 43:1214–1220
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM (2001) HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 108:391–397
Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S (2001) Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 103:2885–2890
Arao K, Yasu T, Umemoto T, Jinbo S, Ikeda N, Ueda S, Kawakami M, Momomura S (2009) Effects of pitavastatin on fasting and postprandial endothelial function and blood rheology in patients with stable coronary artery disease. Circ J 73:1523–1530
Umemoto T, Yasu T, Arao K, Ikeda N, Horie Y, Sugimura H, Kawakami M, Fujita H, Momomura S (2017) Pravastatin improves postprandial endothelial dysfunction and hemorheological deterioration in patients with effort angina pectoris. Heart Vessels. doi:10.1007/s00380-017-0974-7
Landmesser U, Bahlmann F, Mueller M, Spikermann S, Kirschhoff N, Schulz S, Manes C, Fischer D, de Groot K, Fliser D, Fauler G, Marz W, Drexler H (2005) Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 111:2356–2363
Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, Da Ros R, Motz E (2002) Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation 106:1211–1218
Ceriello A, Assaloni R, Da Ros R, Maier A, Piconi L, Quagliaro L, Esposito K, Giugliano D (2005) Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation 111:2518–2524
Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria AN, Investigators REVERSAL (2004) Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 291:1071–1080
Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM, Investigators ASTEROID (2006) Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 295:1556–1565
Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M, Investigators JAPAN-ACS (2009) Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol 54:293–302
Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF (2001) Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 357:577–581
Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, Miche E, Böhm M, Nickenig G (2004) Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109:220–226
Ikeda N, Yasu T, Kubo N, Nakamura T, Sugawara Y, Ueda S, Ishikawa SE, Saito M, Kawakami M, Momomura S (2008) Daily exercise and bone marrow-derived CD34+/133+ cells after myocardial infarction treated by bare metal stent implantation. Circ J 72:897–901
Aoyama N, Nishinari M, Ohtani S, Kanai A, Noda C, Hirata M, Miyamoto A, Watanabe M, Minamino T, Izumi T, Ako J (2017) Clinical features and predictors of patients with critical limb ischemia who responded to autologous mononuclear cell transplantation for therapeutic angiogenesis. Heart Vessels. doi:10.1007/s00380-017-0968-5
Mondillo S, Ballo P, Barbati R, Guerrini F, Ammaturo T, Agricola E, Pastore M, Borrello F, Belcastro M, Picchi A, Nami R (2003) Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med 114:359–364
Mohler ER, Hiatt WR, Creager MA (2003) Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation 108:1481–1486
Harris SK, Roos MG, Landry GJ (2016) Statin use in patients with peripheral arterial disease. J Vasc Surg 64:1881–1888
Feigelson HS, Criqui MH, Fronek A, Langer RD, Molgaard CA (1994) Screening for peripheral arterial disease: the sensitivity, specificity, and predictive value of noninvasive tests in a defined population. Am J Epidemiol 140:526–534
Guo X, Li J, Pang W, Zhao M, Luo Y, Sun Y, Hu D (2008) Sensitivity and specificity of ankle-brachial index for detecting angiographic stenosis of peripheral arteries. Circ J 72:605–610
Arao K, Yasu T, Ohmura N, Tsukamoto Y, Murata M, Kubo N, Umemoto T, Ikeda N, Ako J, Ishikawa S, Kawakami M, Momomura S (2010) Circulating CD34+/133+ progenitor cells in patients with stable angina pectoris undergoing percutaneous coronary intervention. Circ J 74:1929–1935
Gardner AW, Skinner JS, Smith LK (1991) Effects of handrail support on claudication and hemodynamic responses to single-stage and progressive treadmill protocols in peripheral vascular occlusive disease. Am J Cardiol 68:99–105
Lane R, Ellis B, Watson L, Leng GC (2014) Exercise for intermittent claudication. Cochrane Database Syst Rev CD000990. doi:10.1002/14651858.CD000990.pub3
Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN (2002) ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation 106:2055–2060
Steiner S, Niessner A, Ziegler S, Richter B, Seidinger D, Pleiner J, Penka M, Wolzt M, Huber K, Wojta J, Minar E, Kopp CW (2005) Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis 181:305–310
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89:E1–E7
Aung PP, Maxwell HG, Jepson RG, Price JF, Leng GC (2007) Lipid-lowering for peripheral arterial disease of the lower limb. Cochrane Database Syst Rev CD000123. doi:10.1002/14651858.CD000123.pub2
Heart Protection Study Collaborative Group, Emberson J, Bennett D, Link E, Parish S, Danesh J, Armitage J, Collins R (2011) C-reactive protein concentration and the vascular benefits of statin therapy: an analysis of 20,536 patients in the Heart Protection Study. Lancet 377: 469–476
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, JUPITER Study Group (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359:2195–2207
Tani S, Takahashi A, Nagao K, Hirayama A (2015) Contribution of apolipoprotein A-I to the reduction in high-sensitivity C-reactive protein levels by different statins: comparative study of pitavastatin and atorvastatin. Heart Vessels 30:762–770
Yasu T (2009) Differentiation of endothelial progenitor cells: a useful biomarker? Circ J 73:1199–1200
Acknowledgements
This work was partly supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 17790496), and by Grant funding from Kowa Co., Ltd. We wish to thank Sachimi Jinbo for her skillful technical assistance and secretary work.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethical statement
All procedures performed in this study were conducted with the patients’ informed consent, and complied with the national ethical guidelines for medical and health research involving human subjects and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
TY, SM, and MK received honorary fees from Kowa Co., Ltd. The other authors have no financial conflicts of interest to disclose concerning in this study. This work was partly supported by grant funding from Kowa Co., Ltd., which was not involved in the study design, implementation, analysis, or manuscript writing.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Arao, K., Yasu, T., Endo, Y. et al. Effects of pitavastatin on walking capacity and CD34+/133+ cell number in patients with peripheral artery disease. Heart Vessels 32, 1186–1194 (2017). https://doi.org/10.1007/s00380-017-0988-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-017-0988-1