Abstract
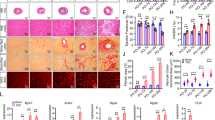
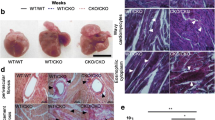
Activin like kinase-1 (AlK-1) mediates signaling via the transforming growth factor beta (TGFβ) family of ligands. AlK-1 activity promotes endothelial proliferation and migration. Reduced AlK-1 activity is associated with arteriovenous malformations. No studies have examined the effect of global AlK-1 deletion on indices of cardiac remodeling. We hypothesized that reduced levels of AlK-1 promote maladaptive cardiac remodeling. To test this hypothesis, we employed AlK-1 conditional knockout mice (cKO) harboring the ROSA26-CreER knock-in allele, whereby a single dose of intraperitoneal tamoxifen triggered ubiquitous Cre recombinase-mediated excision of floxed AlK-1 alleles. Tamoxifen treated wild-type (WT-TAM; n = 5) and vehicle treated AlK-1-cKO mice (cKO-CON; n = 5) served as controls for tamoxifen treated AlK-1-cKO mice (cKO-TAM; n = 15). AlK-1 cKO-TAM mice demonstrated reduced 14-day survival compared to cKO-CON controls (13 vs 100%, respectively, p < 0.01). Seven days after treatment, cKO-TAM mice exhibited reduced left ventricular (LV) fractional shortening, progressive LV dilation, and gastrointestinal bleeding. After 14 days total body mass was reduced, but LV and lung mass increased in cKO-TAM not cKO-CON mice. Peak LV systolic pressure, contractility, and arterial elastance were reduced, but LV end-diastolic pressure and stroke volume were increased in cKO-TAM, not cKO-CON mice. LV AlK-1 mRNA levels were reduced in cKO-TAM, not cKO-CON mice. LV levels of other TGFβ-family ligands and receptors (AlK5, TBRII, BMPRII, Endoglin, BMP7, BMP9, and TGFβ1) were unchanged between groups. Cardiomyocyte area and LV levels of BNP were increased in cKO-TAM mice, but LV levels of β-MHC and SERCA were unchanged. No increase in markers of cardiac fibrosis, Type I collagen, CTGF, or PAI-1, were observed between groups. No differences were observed for any variable studied between cKO-CON and WT-TAM mice. Global deletion of AlK-1 is associated with the development of high output heart failure without maladaptive remodeling. Future studies exploring the functional role of AlK-1 in cardiac remodeling independent of systemic AVMs are required.





Similar content being viewed by others
References
Tual-Chalot S, Oh SP, Arthur HM (2015) Mouse models of hereditary hemorrhagic telangiectasia: recent advances and future challenges. Front Genet 6:25
Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E (2000) Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA 97:2626–2631
Han C, Choe SW, Kim YH, Acharya AP, Keselowsky BG, Sorg BS, Lee YJ, Oh SP (2014) VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis 17:823–830
Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, Oh SH, Walter G, Raizada MK, Sorg BS, Oh SP (2009) Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest 119:3487–3496
Rockman HA, Ono S, Ross RS, Jones LR, Karimi M, Bhargava V, Ross J Jr, Chien KR (1994) Molecular and physiological alterations in murine ventricular dysfunction. Proc Natl Acad Sci USA 91:2694–2698
Kapur NK, Paruchuri V, Aronovitz MJ, Qiao X, Mackey EE, Daly GH, Ughreja K, Levine J, Blanton R, Hill NS, Karas RH (2013) Biventricular remodeling in murine models of right ventricular pressure overload. PLoS One 8:e70802
Kapur NK, Qiao X, Paruchuri V, Mackey EE, Daly GH, Ughreja K, Morine KJ, Levine J, Aronovitz MJ, Hill NS, Jaffe IZ, Letarte M, Karas RH (2014) Reducing endoglin activity limits calcineurin and TRPC-6 expression and improves survival in a mouse model of right ventricular pressure overload. J Am Heart Assoc 3:e000965
Kapur NK, Wilson S, Yunis AA, Qiao X, Mackey E, Paruchuri V, Baker C, Aronovitz MJ, Karumanchi SA, Letarte M, Kass DA, Mendelsohn ME, Karas RH (2012) Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation 125:2728–2738
Fukui M, Goda A, Komamura K, Nakabo A, Masaki M, Yoshida C, Hirotani S, Lee-Kawabata M, Tsujino T, Mano T, Masuyama T (2016) Changes in collagen metabolism account for ventricular functional recovery following beta-blocker therapy in patients with chronic heart failure. Heart Vessels 31:173–182
Harada M, Hojo M, Kamiya K, Kadomatsu K, Murohara T, Kodama I, Horiba M (2016) Exogenous midkine administration prevents cardiac remodeling in pacing-induced congestive heart failure of rabbits. Heart Vessels 31:96–104
Ikeda Y, Inomata T, Fujita T, Iida Y, Nabeta T, Ishii S, Maekawa E, Yanagisawa T, Mizutani T, Naruke T, Koitabashi T, Takeuchi I, Ako J (2016) Cardiac fibrosis detected by magnetic resonance imaging on predicting time course diversity of left ventricular reverse remodeling in patients with idiopathic dilated cardiomyopathy. Heart Vessels 31:1817–1825
Garcia-Tsao G, Korzenik JR, Young L, Henderson KJ, Jain D, Byrd B, Pollak JS, White RI Jr (2000) Liver disease in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 343:931–936
Buscarini E, Leandro G, Conte D, Danesino C, Daina E, Manfredi G, Lupinacci G, Brambilla G, Menozzi F, De Grazia F, Gazzaniga P, Inama G, Bonardi R, Blotta P, Forner P, Olivieri C, Perna A, Grosso M, Pongiglione G, Boccardi E, Pagella F, Rossi G, Zambelli A (2011) Natural history and outcome of hepatic vascular malformations in a large cohort of patients with hereditary hemorrhagic teleangiectasia. Dig Dis Sci 56:2166–2178
Dupuis-Girod S, Ginon I, Saurin JC, Marion D, Guillot E, Decullier E, Roux A, Carette MF, Gilbert-Dussardier B, Hatron PY, Lacombe P, Lorcerie B, Rivière S, Corre R, Giraud S, Bailly S, Paintaud G, Ternant D, Valette PJ, Plauchu H, Faure F (2012) Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA 307:948–955
Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, Spears J, Brown DH, Buscarini E, Chesnutt MS, Cottin V, Ganguly A, Gossage JR, Guttmacher AE, Hyland RH, Kennedy SJ, Korzenik J, Mager JJ, Ozanne AP, Piccirillo JF, Picus D, Plauchu H, Porteous ME, Pyeritz RE, Ross DA, Sabba C, Swanson K, Terry P, Wallace MC, Westermann CJ, White RI, Young LH, Zarrabeitia R, HHT Foundation International—Guidelines Working Group (2011) International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 48:73–87
Garcia-Tsao G (2007) Liver involvement in hereditary hemorrhagic telangiectasia (HHT). J Hepatol 46:499–507
Gincul R, Lesca G, Gelas-Dore B, Rollin N, Barthelet M, Dupuis-Girod S, Pilleul F, Giraud S, Plauchu H, Saurin JC (2008) Evaluation of previously nonscreened hereditary hemorrhagic telangiectasia patients shows frequent liver involvement and early cardiac consequences. Hepatology 48:1570–1576
Ginon I, Decullier E, Finet G, Cordier JF, Marion D, Saurin JC, Dupuis-Girod S (2013) Hereditary hemorrhagic telangiectasia, liver vascular malformations and cardiac consequences. Eur J Intern Med 24:e35–e39
Lerut J, Orlando G, Adam R, Sabbà C, Pfitzmann R, Klempnauer J, Belghiti J, Pirenne J, Thevenot T, Hillert C, Brown CM, Gonze D, Karam V, Boillot O, Association European Liver Transplant (2006) Liver transplantation for hereditary hemorrhagic telangiectasia: report of the European liver transplant registry. Ann Surg 244:854–862
Karram T, Hoffman A, Bishara B, Brodsky S, Golomb E, Winaver J, Abassi Z (2005) Induction of cardiac hypertrophy by a controlled reproducible sutureless aortocaval shunt in the mouse. J Invest Surg 18:325–334
Scheuermann-Freestone M, Freestone NS, Langenickel T, Höhnel K, Dietz R, Willenbrock R (2001) A new model of congestive heart failure in the mouse due to chronic volume overload. Eur J Heart Fail 3:535–543
Braun MU, LaRosée P, Simonis G, Borst MM, Strasser RH (2004) Regulation of protein kinase C isozymes in volume overload cardiac hypertrophy. Mol Cell Biochem 262:135–143
Calderone A, Takahashi N, Izzo NJ Jr, Thaik CM, Colucci WS (1995) Pressure- and volume-induced left ventricular hypertrophies are associated with distinct myocyte phenotypes and differential induction of peptide growth factor mRNAs. Circulation 92:2385–2390
Boillot O, Bianco F, Viale JP, Mion F, Mechet I, Gille D, Delaye J, Paliard P, Plauchu H (1999) Liver transplantation resolves the hyperdynamic circulation in hereditary hemorrhagic telangiectasia with hepatic involvement. Gastroenterology 116:187–192
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Morine, K.J., Qiao, X., Paruchuri, V. et al. Conditional knockout of activin like kinase-1 (ALK-1) leads to heart failure without maladaptive remodeling. Heart Vessels 32, 628–636 (2017). https://doi.org/10.1007/s00380-017-0955-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-017-0955-x