Abstract
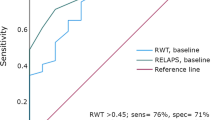
Wild-type transthyretin amyloidosis (ATTRwt) is often overlooked in elderly patients with left ventricular hypertrophy (LVH). Impaired atrial function, in addition to ventricular diastolic dysfunction, is one of the hallmarks of cardiac amyloidosis. Here, we assessed the hypothesis that atrial function evaluated by A-velocity in pulse Doppler echocardiography is useful to differentiate ATTRwt in elderly patients with LVH. We analyzed 133 consecutive patients who underwent tissue biopsy to rule out infiltrative cardiomyopathy in our institute. We excluded patients younger than 50 years, without LVH (LV thickness was less than 12 mm), with other types of cardiac amyloidosis and patients with chronic atrial fibrillation, and analyzed remaining 51 patients (ATTRwt: 16, non-ATTRwt: 35). ATTRwt patients were significantly older and had advanced heart failure compared with non-ATTRwt group. In echocardiography, E/A, E/e′, and relative wall thickness was significantly higher in ATTRwt group than non-ATTRwt group. A-velocity was significantly decreased in ATTRWT group compared with non-ATTRwt group (40.8 ± 20.8 vs. 78.7 ± 28.2 cm/s, p = 0.0001). Multivariate logistic analysis using eight forced inclusion models identified trans-mitral Doppler A-wave velocity was more significant factor of cardiac amyloidosis in ATTRwt. In receiver operating characteristic (ROC) analysis, the area under the curve (AUC) for A-wave velocity in discrimination between ATTRwt and non-ATTRwt were 0.86 (CI 0.76–0.96, p < 0.001). The cut-off value was 62.5 cm/s, and it yielded the best combination of sensitivity (69.7%) and specificity (87.5%) for prediction of amyloidosis. We concluded that reduced A-velocity predicts the presence of ATTRwt in elderly patients with LVH in sinus rhythm.


Similar content being viewed by others
References
Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Myllykangas L (2008) Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and τ: a population-based autopsy study. Ann Med 40:232–239
Rahman JE, Helou EF, Gelzer-Bell R, Thompson RE, Kuo C, Rodriguez ER, Kasper EK (2004) Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol 43:410–415
Plehn JF, Southworth J, Cornwell GG III (1992) Atrial systolic failure in primary amyloidosis. N Engl J Med 327:1570–1573
Dubrey S, Pollak A, Skinner M, Falk RH (1995) Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: evidence for atrial electromechanical dissociation. Br Heart J 74:541–544
Kwong RY, Heydari B, Abbasi S, Steel K, Al-Mallah M, Wu H, Falk RH (2015) Characterization of cardiac amyloidosis by atrial late gadolinium enhancement using contrast-enhanced cardiac magnetic resonance imaging and correlation with left atrial conduit and contractile function. Am J Cardiol 116:622–629
Migrino RQ, Harmann L, Christenson R, Hari P (2014) Clinical and imaging predictors of 1-year and long-term mortality in light chain (AL) amyloidosis: a 5-year follow-up study. Heart Vessels 29:793–800
Hashimura H, Ishibashi UH, Yonemoto Y, Ohta OK, Matsuyama TA, Ikeda Y, Morita Y, Yamada N, Yasui H, Naito H (2016) Late gadolinium enhancement in cardiac amyloidosis: attributable both to interstitial amyloid deposition and subendocardial fibrosis caused by ischemia. Heart Vessels 31:990–995
Modesto KM, Dispenzieri A, Cauduro SA, Lacy M, Khandheria BK, Pellikka PA, Gertz M (2005) Left atrial myopathy in cardiac amyloidosis: implications of novel echocardiographic techniques. Eur Heart J 26:173–179
Moyssawtkis I, Triposkiadis F, Pantazopoulos NJ, Kyriakidis M, Nihoyannopoulos P (2004) Left atrial systolic function in primary and familial amyloidosis: assessment from left atrial volume change. Clin Cardiol 27:528–532
Murphy L, Falk RH (2000) Left atrial kinetic energy in AL amyloidosis: can it detect early dysfunction? Am J Cardiol 86:244–246
Manning WJ, Silverman DI, Katz SE, Riley MF, Doherty RM, Munson JT, Douglas PS (1995) Temporal dependence of the return of atrial mechanical function on the mode of cardioversion of atrial fibrillation to sinus rhythm. Am J Cardiol 75:624–626
Leung DY, Boyd A, Ng AA, Chi C, Thomas L (2008) Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J 156:1056–1064
Klein AL, Hatle LK, Taliercio C, Oh JK, Kyle RA, Gertz MA, Tajik AJ (1991) Prognostic significance of Doppler measures of diastolic function in cardiac amyloidosis. A Doppler echocardiography study. Circulation 83:808–816
Klein AL, Hatle LK, Taliercio CP, Taylor CL, Kyle RA, Bailey KR, Tajik AJ (1990) Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol 16:1135–1141
Klein AL, Burstow DJ, Tajik AJ, Zachariah PK, Bailey KR, Seward JB (1994) Effects of age on left ventricular dimensions and filling dynamics in 117 normal persons. Mayo Clin Proc 69:212–224
Yu CM, Sanderson JE, Marwick TH, Oh JK (2007) Tissue Doppler imaging: a new prognosticator for cardiovascular diseases. J Am Coll Cardiol 49:1903–1914
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’for medical statistics. Bone Marrow Transplant 48:452–458
Falk RH (2005) Diagnosis and management of the cardiac amyloidoses. Circulation 112:2047–2060
Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S (2007) Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol 50:2101–2110
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the ethics committee of Kumamoto University Hospital and was populated in accordance with the Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the study.
Conflict of interest
The authors have declared no conflicts of interest.
Rights and permissions
About this article
Cite this article
Yamamura, S., Izumiya, Y., Ishida, T. et al. Reduced trans-mitral A-wave velocity predicts the presence of wild-type transthyretin amyloidosis in elderly patients with left ventricular hypertrophy. Heart Vessels 32, 708–713 (2017). https://doi.org/10.1007/s00380-016-0925-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-016-0925-8