Abstract
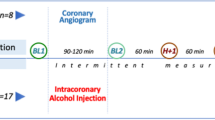
We have developed a porcine model of acute myocardial infarction (AMI) and ischemic heart failure by transcatheter intracoronary injection of ethyl alcohol and observed pathologic changes induced in the alcohol-injured coronary artery and infarcted myocardium. In a total of 12 female pigs, anteroseptal AMI was induced by transcatheter delivery of 1 mL of 99.9% ethyl alcohol using a 2.5 mm diameter over-the-wire balloon catheter in the left anterior descending artery (LAD). Another five pigs underwent the sham operation, and the differences in left ventricular (LV) dimension and LV ejection fraction between these pigs and those injected with ethyl alcohol were evaluated. Follow-up coronary and LV angiography, echocardiography and histopathology were performed at 4 weeks after the procedure. Myocardial SPECT using 201Tl (and 99mTc-MIBI) and triphenyl tetrazolium chloride (TTC) stain were performed and compared. Procedure-related death occurred in two pigs with proximal LAD occlusion. Four pigs suffered from ventricular tachycardia, which converted to sinus rhythm by DC cardioversion. Follow-up coronary angiography at 4 weeks revealed persistent total occlusion in all pigs. Echocardiogram showed decreased apicoanteroseptal wall motion with an ejection fraction of 46.5 ± 3.3% and nonsignificantly changed LV dimensions. Myocardial SPECT revealed a perfusion defect in the apicoanterior wall in all subjects (percent area of the perfusion defect = 22.1 ± 2.50%). The percentage of myocardium not stained by TTC was 23.1 ± 2.25%. Histologic examination revealed severe fibrosis in the infarcted myocardium and massive thrombus with organization and calcification in the alcohol-injured coronary artery. The porcine model of AMI obtained by intracoronary alcohol injection provides a safe and reproducible method for the research and development of new therapeutic modalities for MI and end-stage heart failure.




Similar content being viewed by others
References
Pfeffer MA, Braunwald E (1990) Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation 81:1161–1172
The TIMI study group (1985) The thrombolysis in myocardial infarction (TIMI) trial: phase I findings. N Engl J Med 312:932–936
Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, Higashino Y, Fujii K, Minamino T (1996) Clinical implication of the “no reflow” phenomenon. Circulation 93:223–228
Korbling A, Estrov Z (2003) Medical progress: adult stem cells for tissue repair—a new therapeutic concept? Lancet 349:570–582
Strauer B, Kornowski R (2003) Stem cell therapy in perspective. Circulation 107:929–934
Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T (2001) Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 104:1046–1052
Ayisi K, Darup J, Krebber HJ, Rodewald G, Kuck KH (1989) Alcohol-induced coagulation necrosis in cardiac tissue: a new concept in the surgical management of recurrent ventricular arrhythmias. Thorac Cardiovasc Surg 37:76–79
Weismuller P, Mayer U, Richter P, Heieck F, Kochs M, Hombach V (1991) Chemical ablation by subendocardial injection of ethanol via catheter-preliminary results in the pig heart. Eur Heart J 12:1234–1239
Haines DE, Whayne JG, DiMarco JP (1994) Intracoronary ethanol ablation in swine: effects of ethanol concentration on lesion formation and response to programmed ventricular stimulation. J Cardiovasc Electrophysiol 5:422–431
Sahn DJ, DeMaria A, Kisslo J, Weyman A (1978) Recommendation regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 68:1072–1083
Maxwell MP, Hears DJ, Yellon DM (1987) Species variation in the coronary collateral circulation during regional myocardial ischemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res 21:737–746
Bertho E, Gagnon G (1964) A comparative study in three dimensions of the blood supply of the normal interventricular septum in human, canine, bovine, porcine, ovine and equine heart. Dis Chest 46:251–262
Markovitz LJ, Savage EB, Ratcliffe MB, Bavaria JE, Kreiner G, Iozzo RV, Hargrove WC 3rd, Bogen DK, Edmunds LH Jr (1989) Large animal model of left ventricular aneurysm. Ann Thorac Surg 48:838–845
Millner RW, Mann JM, Pearson I, Pepper JR (1991) Experimental model of left ventricular failure. Ann Thorac Surg 52:78–83
Terp K, Kim WY, Ulrich M, Frokiaer J, Baandrup U, Rehling M, Bagger JP, Hasenkam JM (1998) The hemodynamic impact of diffuse myocardial ischemic lesions: an animal experimental model based on intracoronary microembolization. Heart Vessels 13:132–141
Harada K, Grossman W, Friedman M, Edelman ER, Prasad PV, Keighley CS, Manning WJ, Sellke FW, Simons M (1994) Basic fibroblast growth factor improves myocardial function in chronically ischemic porcine hearts. J Clin Invest 94:623–630
Mack CA, Patel SR, Schwarz EA, Zanzonico P, Hahn RT, Ilercil A, Devereux RB, Goldsmith SJ, Christian TF, Sanborn TA, Kovesdi I, Hackett N, Isom OW, Crystal RG, Rosengart TK (1998) Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor 121 improves myocardial perfusion and function in the ischemic porcine heart. J Thorac Cardiovasc Surg 115:168–177
Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S (1991) A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol 260:1379–1384
Li RK, Weisel RD, Mickle DA, Jia ZQ, Kim EJ, Sakai T, Tomita S, Schwartz L, Iwanochko M, Husain M, Cusimano RJ, Burns RJ, Yau TM (2000) Autologous porcine heart cell transplantation improved heart function after a myocardial infarction. J Thorac Cardiovasc Surg 119:62–68
Sakaguchi G, Sakakibara Y, Tambara K, Lu F, Premaratne G, Nishimura K, Komeda M (2003) A pig model of chronic heart failure by intracoronary embolization with gelatin sponge. Ann Thorac Surg 75:1942–1947
Maron BJ (2000) Role of alcohol septal ablation in treatment of obstructive hypertrophic cardiomyopathy. Lancet 355:425–426
Li ZQ, Cheng TO, Liu L, Jin YZ, Zhang M, Guan RM, Yuan L, Hu J, Zhang WW (2003) Experimental study of relationship between intracoronary alcohol injection and the size of resultant myocardial infarct. Int J Cardiol 91:93–96
Veselka J, Zemánek D, Tomasov P, Duchonová R, Linhartová K (2009) Alcohol septal ablation for obstructive hypertrophic cardiomyopathy: ultra-low dose of alcohol (1 ml) is still effective. Heart Vessels 24:27–31
Duzenli MA, Ozdemir K, Aygul N, Soylu A, Aygul MU, Gök H (2009) Comparison of myocardial performance index obtained either by conventional echocardiography or tissue Doppler echocardiography in healthy subjects and patients with heart failure. Heart Vessels 24:8–15
Hua W (1992) The pathologic myocardial change caused by intracoronary ethyl alcohol injection and its mechanism. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 14:371–373
Acknowledgments
This work was supported by the Bio R&D program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (No. 2010-0019913).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, W., Jeong, M.H., Sim, D.S. et al. A porcine model of ischemic heart failure produced by intracoronary injection of ethyl alcohol. Heart Vessels 26, 342–348 (2011). https://doi.org/10.1007/s00380-010-0022-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-010-0022-3