Abstract
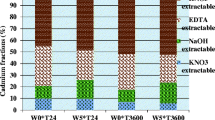
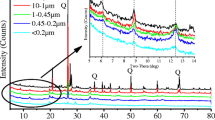
The size fraction of soils is one of the important factors that influence the retention of heavy metals. The sorptive properties of soils for heavy metals are principally associated with clay and silt-size fractions. Phosphate fertilizers that are applied to highly weathered tropical soils contain a wide concentration range of cadmium (Cd) as an impurity. Tropical soils contain kaolinite and oxides of Al, Fe, and Mn, which have the ability to sorb Cd. However, the distribution of Cd in different size fractions and the chemical speciation of particulate-bound Cd in the clay size fractions when introduced to soil and allowed to incubate at field moisture capacity merits attention. Cadmium was, therefore, applied to selected surface Kenyan soils varying widely in physicochemical properties to investigate its distribution in different soil particle size fractions and the speciation of particulate-bound species in clay size fractions after incubation. The Cd content in different particle fractions was analyzed by graphite furnace atomic absorption technique after HF-HClO4 digestion. The particulate-bound Cd species were investigated using chemical sequential extraction method. The study showed that clay size fraction of the natural and the Idaho monoammonium phosphate (MAP)-fertilizer or the Cd perchlorate-added MAP chemical reagent-treated soils contained the highest amount of the total Cd. However, silt and sand fractions of the treated soils also retained appreciable amounts of Cd. Speciation studies revealed that metal-organic complex-bound Cd was the most predominant compared to other particulate-bound Cd species in the clay size fractions of the soils treated with Idaho MAP fertilizer or the Cd perchlorate-added MAP chemical reagent. The distribution of total Cd in the different soil particle size fractions and the speciation of particulate-bound Cd in the clay size fractions varied with the soil type. The results indicate that clay size fractions can retain Cd making it less available; however, the influence of farming practices, which affect Cd mobility, should not be overlooked.


Similar content being viewed by others
References
Alloway BJ (1995) Cadmium. In: Alloway BJ (ed) Heavy metals in soils, 2nd edn. Blackie Academic & Professional, London, pp 122–151
Alloway BJ, Steinnes E (1999) Anthropogenic additions of cadmium to soils. In: McLaughlin MJ, Singh BR (eds) Cadmium in soils and plants. Kluwer Academic, Dordrecht, The Netherlands, pp 97–123
Annual Farmers’ Recommendations (1993) Kenya agricultural research institute, plant breeding research centre. Njoro, Kenya
Christensen TM, Huang PM (1999) Solid phase cadmium and the reactions of aqueous cadmium with soil surfaces. In: McLaughlin MJ, Singh BR (eds) Cadmium in soils and plants. Kluwer Academic, Dordrecht, The Netherlands, pp 65–96
Dixon JB, White GN (2002) Manganese oxides. In: Dixon JB, Schulze DG (eds) Soil mineralogy with environmental applications, SSSA book series 7. Soil Sci Sol Am, Madison, WI, pp 367–388
Jackson ML (1958) Soil chemical analysis. Prentice Hall, Englewood Cliff, NJ, pp 498
Jackson ML (1979) Soil chemical analysis—advanced course. Department of Soil Science, University of Wisconsin, Madison, WI, 895 pp (Published by the author)
Jones LHP, Jarvis SC (1981) The fate of heavy metals. In: Greenland DJ, Hayes MHB (ed), The chemistry of soil processes. Wiley, New York, pp 593–620 (a Wiley–Interscience Publication)
Krishnamurti GSR, Huang PM (1987) Influence of constituents on the stability of mechanical separates of soils representing major taxonomic orders. Appl Clay Sci 2:299–308
Krishnamurti GSR, Huang PM, Van Rees KCJ, Kozak LM, Rostad HPW (1994) Microwave digestion techniques for the determination of total cadmium in soils. Commun Soil Sci Plant Anal 25:615–625
Krishnamurti GSR, Huang PM, Van Rees KCJ, Kozak LM, Rostad HPW (1995) Speciation of particulate-bound cadmium of soils and bioavailability. Analyst 120:659–665
Mann SS, Ritchie GSP (1994) Changes in the forms of cadmium with time in some Western Australian soils. Aust J Soil Res 32:241–250
McArthur DFE (2001) Impact of long-term cultivation on the status of cadmium in chernozemic soils. Ph.D. thesis, University of Saskatchewan, Saskatoon, SK
McKeague JA (1967) An evaluation of 0.1 M pyrophosphate and pyrophosphate-dithionite in comparison with oxalate as extractants of the accumulation products in podozols and some other soils. Can J Soil Sci 47:95–99
McKeague JA, Day JH (1966) Dithionite and oxalate extractable Fe and Al as aids in differentiating various classes of soils. Can J Soil Sci 46:13–22
McKenzie RM (1989) Manganese oxides and hydroxides. In: Dixon JB, Weed SB (eds) Minerals in soil environments. Soil Sci Soc Am, Madison, WI, pp 439–465
Mehra OP, Jackson ML (1960) Iron oxide removal from soils by dithionite-citrate system buffered with sodium bicarbonate. Clays Clay Miner 7:319–327
Onyatta JO (1997) Kinetics and equilibria of cadmium in selected Kenyan soils. Ph.D. thesis, University of Saskatchewan, Saskatoon, SK
Onyatta JO, Huang PM (1999) Chemical speciation and bioavailability index of cadmium for selected tropical soils in Kenya. Geoderma 91:87–101
Pendias AK, Pendias H (1984) Trace metals in soils and plants. CRC Press, Boca Raton, Fl, p 315
Pickering WF (1980) Cadmium retention by clays and other soil or sediment Components. In: Nriagu JO (ed) Cadmium in the environment. Part I. Ecological cycling. John Wiley, New York, pp 365–395
Puls RW, Bohn HL (1988) Sorption of cadmium, nickel and zinc by kaolinite and montmorillonite suspensions. Soil Sci Soc Am J 52:1289–1292
Sposito G (1977) Soil chemistry. Encyclopedia of science and technology, 4th edn. McGraw-Hill, New York
Sullivan PJ (1977) The principle of hard and soft acids and bases as applied to exchangeable cation selectivity in soils. Soil Sci 124:117–121
Wagann MR, Levi-Minzi R, Riffaldi R (1978) The effect of P source, time, moisture, and pH on the movement of Cd in soils. Z Pflanzenernähr Bodenkd 141:241–249
Wang D, Anderson D (1998) Direct determination of organic carbon content in soils by Leco 12 carbon analyzer. Commun Soil Sci Plant Anal 29:15–21
Welch RM, Norvell WA (1999) Mechanisms of cadmium uptake, translocation and deposition in plants. In: McLaughlin MJ, Singh BR (eds) Cadmium in soils and plants. Kluwer Academic, Dordrecht, The Netherlands, pp 125–150
Acknowledgements
The Canadian International Development Agency (CIDA) and Discovery Grant GP 2383-Huang of the Natural Sciences and Engineering Research Council of Canada supported this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onyatta, J.O., Huang, P.M. Distribution of applied cadmium in different size fractions of soils after incubation. Biol Fertil Soils 42, 432–436 (2006). https://doi.org/10.1007/s00374-006-0087-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-006-0087-4