Abstract
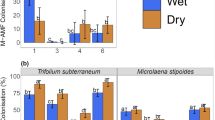
Intracellular arbuscular mycorrhizal (AM) colonization was compared between nitrogen (NH4NO3) fertilized (10 g N m−2) and nonfertilized tallgrass prairie plots. In the microscopic analyses of host roots, only intracellular coils showed an increasing trend as a result of N fertilization, whereas intracellular colonization by arbuscules, hyphae, or vesicles did not differ between the N treatments. Clone libraries established from pooled PCR products of AM fungi contained exclusively species of Glomus; no other genera were detected indicating that Glomus spp. dominated the host roots. Comparisons between observed and random topologies indicated that cloned sequence placement covaried with N treatment: unique clades within Glomus originated exclusively from N-fertilized or nonfertilized treatments. We conclude that the communities of dominant and most commonly occurring AM fungi changed in response to N amendment, although the root colonization showed minimal or no response.



Similar content being viewed by others
References
Aerts R (2002) The role of various types of mycorrhizal fungi in nutrient cycling and plant competition. In: van der Heijden MGA, Sanders I (eds) Mycorrhizal ecology. Springer, Berlin Heidelberg New York, pp 117–133
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Avis PG, McLaughlin DJ, Dentinger BC, Reich PB (2003) Long-term increase in nitrogen supply alters above- and below-ground ectomycorrhizal communities and increases the dominance of Russula spp. in a temperate oak savanna. New Phytol 160:239–253
Bentivenga SP, Hetrick BAD (1992a) The effect of prairie management practices on mycorrhizal symbiosis. Mycologia 84:522–527
Bentivenga SP, Hetrick BAD (1992b) Seasonal and temperature effects on mycorrhizal activity and dependence of cool- and warm-season tallgrass prairie grasses. Can J Bot 70:1596–1602
Borneman J, Hartin RJ (2000) PCR primers that amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol 66:4356–4360
Clapp JP, Young JPW, Merryweather JW, Fitter AH (1995) Diversity of fungal symbionts in arbuscular mycorrhizas from a natural community. New Phytol 130:259–265
Egerton-Warburton LM, Allen EB (2000) Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol Appl 10:484–496
Egerton-Warburton LM, Graham RC, Allen EB, Allen MF (2001) Reconstruction of the historical changes in mycorrhizal fungal communities under anthropogenic nitrogen deposition. Proc R Soc Lond Ser B 268:2479–2484
Eom A-H, Hartnett DC, Wilson GT, Figge DAH (1999) The effect of fire, mowing and fertilizer amendment on arbuscular mycorrhizas in tallgrass prairie. Am Midl Nat 142:55–70
Eom A-H, Hartnett DC, Wilson GWT (2000) Host plant species effects on arbuscular mycorrhizal communities in tallgrass prairie. Oecologia 122:435–444
Eom A-H, Wilson GWT, Hartnett DC (2001) Effects of ungulate grazers on arbuscular mycorrhizal symbiosis and fungal community structure in tallgrass prairie. Mycologia 93:233–242
Fenn ME, Poth MA, Aber JD, Baron JS, Borman BT, Johnson DW, Lemly AD, McNulty SG, Ryan DF, Stottlemeyr R (1998) Nitrogen excess in North American ecosystems: predisposing factors, ecosystem responses and management strategies. Ecol Appl 8:706–733
Ford JE, McHeyzer-Williams MG, Lieber MR (1994) Chimeric molecules created by gene amplification interfere with the analyses of somatic hypermutation of murine immunoglobulin genes. Gene 142:279–283
Gardes M, Bruns TD (1996) ITS-RFLP matching for the identification of fungi. In: Clapp JP (eds) Methods in molecular biology, vol 50. Species diagnostics protocols: PCR and other nucleic acid methods. Humana Press, Totowa, NJ, pp 177–186
Hartnett DC, Wilson GWT (1999) Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80:1187–1195
Hayman DS (1970) Endogone spore numbers in soil and vesicular arbuscular mycorrhiza in wheat as influenced by season and soil treatment. Trans Br Mycol Soc 54:53–63
Hayman DS (1982) Influence of soils and fertility on activity and survival of vesicular-arbuscular mycorrhizal fungi. Phytopathology 72:1119–1125
Helgason T, Daniell TJ, Husband R, Fitter AH, Young JPW (1998) Ploughing up the wood-wide web? Nature 394:431
Helgason T, Fitter AH, Young JPW (1999) Molecular diversity of arbuscular mycorrhizal fungi colonizing Hyacinthoides non-scripta (bluebell) in a seminatural woodland. Mol Ecol 8:659–666
Helgason T, Merryweather JW, Denison J, Wilson P, Young JPW, Fitter AH (2002) Selectivity and functional diversity in arbuscular mycorrhizas of co-occurring fungi and plants from a temperate deciduous woodland. J Ecol 90:371–384
Husband R, Herre EA, Turner SL, Gallery R, Young JPW (2002) Molecular diversity of arbuscular mycorrhizal fungi and patterns of host association over time and space in a tropical forest. Mol Ecol 11:2669–2678
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1895–1908
Jumpponen A (2003) Soil fungal community assembly in a primary successional glacier forefront ecosystem as inferred from rDNA sequence analyses. New Phytol 158:569–578
Kochy M, Wilson SD (2001) Nitrogen deposition and forest expansion in the northern Great Plains. J Ecol 89:807–817
Kopczynski ED, Bateson MM, Ward DM (1994) Recognition of chimeric small-subunit ribosomal DNAs composed from genes from uncultivated microorganisms. Appl Environ Microbiol 63:3614–3621
Lilleskov EA, Fahey TJ, Lovett GM (2001) Ectomycorrhizal fungal aboveground community change over an atmospheric nitrogen deposition gradient. Ecol Appl 11:2001
Lilleskov EA, Fahey TJ, Horton TR, Lovett GM (2002) Belowground ectomycorrhizal community change over a nitrogen deposition gradient in Alaska. Ecology 83:104–115
Lloyd-MacGilp SA, Chambers SM, Dodd JC, Fitter AH, Walker C, Young JPW (1996) Diversity of the ribosomal internal transcribed spacers within and among isolates of Glomus mosseae and related mycorrhizal fungi. New Phytol 133:103–111
Maddison DR, Maddison WP (2001) MacClade 4: analysis of phylogeny and character evolution. Version 4.06. Sinauer, Sunderland, MA
Maddison WP, Slatkin M (1991) Null models for the number of evolutionary steps in a character on a phylogenetic tree. Evolution 45:1184–1197
Martin AP (2002) Phylogenetic approaches for describing and comparing the diversity of microbial communities. Appl Environ Microbiol 68:3673–3682
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Merryweather J, Fitter A (1998) The arbuscular mycorrhizal fungi of Hyacinthoides non-scripta I. Diversity of fungal taxa. New Phytol 138:117–129
Morton JB, Redercker D (2001) Two new families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93:181–195
Morton JB, Bentivenga SP, Bever JD (1995) Discovery, measurement, and interpretation of diversity in arbuscular endomycorrhizal fungi (Glomales, Zygomycetes). Can J Bot 73:S25–S32
Peter M, Ayer F, Egli S (2001) Nitrogen addition in a Norway spruce stand altered macromycete sporocarp production and below-ground ectomycorrhizal species composition. New Phytol 149:311–325
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Rillig MC, Treseder KK, Allen MF (2002) Global change and mycorrhizal fungi. In: van der Heijden MGA, Sanders I (eds) Mycorrhizal ecology. Springer, Berlin Heidelberg New York, pp 135–160
Sambrook J (1989) Molecular cloning—a laboratory manual. Cold Spring Laboratory Press, New York
Sanders IR, Fitter AH (1992) The ecology and functioning of vesicular-arbuscular mycorrhizas in co-existing grassland species. I. Seasonal patterns of mycorrhizal occurrence and morphology. New Phytol 120:525–533
Simon L, Lalonde M, Bruns TD (1992) Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl Environ Microbiol 58:291–295
Swofford DL (2001) PAUP*, Phylogenetic analysis using parsimony (and other methods). Version 4. Sinauer, Sunderland, MA
Sylvia DM, Neal LH (1990) Nitrogen affects the phosphorus response of VA mycorrhiza. New Phytol 115:303–310
Vandenkoornhuyse P, Husband R, Daniell TJ, Watson IJ, Duck JM, Fitter AH, Young JPW (2002) Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Mol Ecol 11:1555–1564
van der Heijden MGA, Boller T, Wiemken A, Sanders IA (1998a) Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79:2082–2091
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998b) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
van der Heijden MGA, Wiemken A, Sanders IA (2003) Different arbuscular mycorrhizal fungi alter co-existence and resource distribution between co-occurring plants. New Phytol 157:569–578
Vitousek P, Aber J, Howart R, Likens G, Matson P, Schindler D, Schlesinger W, Tilman D (1997a) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997b) Human domination of earth’s ecosystems. Science 277:494–499
von Wintzingerode F, Göbel UB, Stackebrandt E (1997) Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev 21:213–229
Wallenda T, Kottke I (1998) Nitrogen deposition and ectomycorrhizas. New Phytol 139:169–187
Wang GCY, Wang Y (1996) The frequency of chimeric molecules as a consequence of PCR co-amplification of 16S rRNA genes from different bacterial species. Microbiology 142:1107–1114
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Acknowledgements
This work was partially supported by Kansas State University BRIEF program and EPSCoR grant No. 9874732 with matching support from the State of Kansas and National Science Foundation (DEB-0344838) (to A.J.). Justin Trowbridge was supported by Konza Prairie LTER “Research Experiences for Undergraduates (REU) program” (National Science Foundation DBI-0243890 to B.K. Sandercock, W.K. Dodds, K.B. Gido, and G.W. Wilson): Conservation of the tallgrass prairie. Konza Prairie Biological Station (KPBS) maintained the field sites and was supported by National Science Foundation Long Term Ecological Research Program (LTER). Louise Egerton-Warburton, Charles L. Kramer, Nicholas B. Simpson, and two anonymous reviewers provided helpful comments on early drafts of this manuscript
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jumpponen, A., Trowbridge, J., Mandyam, K. et al. Nitrogen enrichment causes minimal changes in arbuscular mycorrhizal colonization but shifts community composition—evidence from rDNA data. Biol Fertil Soils 41, 217–224 (2005). https://doi.org/10.1007/s00374-005-0845-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-005-0845-8